Table of Contents
The search for the dawn of life has long captivated the scientific community, with one intriguing hypothesis suggesting that RNA, or ribonucleic acid, played a pivotal role before the era of DNA-based life. RNA, still a fundamental component of life today, possesses the remarkable ability to replicate itself and catalyze various chemical reactions. This article delves into the complex world of early Earth chemistry, exploring the challenges and breakthroughs in understanding how RNA and its building blocks, ribonucleotides, may have originated.
RNA and the Building Blocks Challenge
RNA molecules are composed of smaller components known as ribonucleotides. The fundamental question arises: how did these building blocks come into existence on the primitive Earth and eventually combine to form RNA?
Chemists are at the forefront of this investigation, attempting to recreate the intricate chain of reactions required for RNA formation under the messy and complex conditions prevalent billions of years ago.
Autocatalysis: A Key Player in the Chemical Orchestra
- One avenue of exploration involves autocatalytic reactions, where chemicals produced encourage the same reaction to happen again, allowing the process to sustain itself.
- Autocatalysis is no stranger to the biological realm, playing vital roles in everything from regulating heartbeats to forming patterns in seashells.
- The replication of life itself is a remarkable example of autocatalysis, as one cell consumes nutrients and energy from the environment to produce two cells.
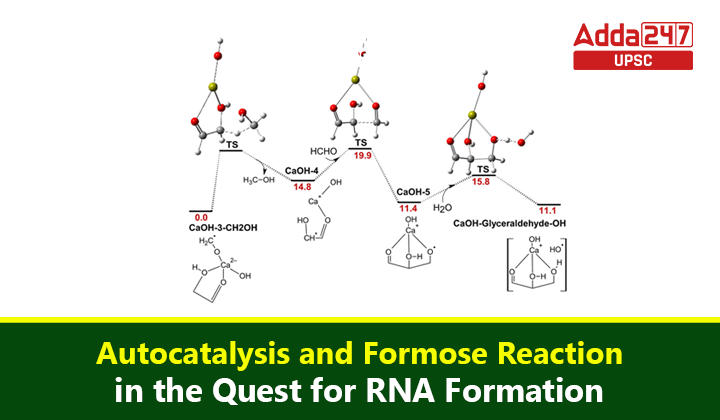
Formose Reaction
- At the heart of this exploration is the foremost reaction, a classic example of an autocatalytic reaction that could have transpired on early Earth.
- Discovered in 1861, the formose reaction involves the transformation of a simple compound, glycolaldehyde, into a more complex molecule.
- This reaction depends on a constant supply of another compound, formaldehyde, and exhibits autocatalytic characteristics.
Challenges of Formose Reaction
- Despite its autocatalytic nature, the formose reaction has its challenges, primarily its notorious lack of selectivity. The reaction produces a plethora of molecules, including many undesired ones, alongside the target products.
- This inherent lack of selectivity has posed a significant obstacle to linking the formose reaction to the established pathway for ribonucleotide production known as the Powner-Sutherland pathway.
Autocatalytic Twist: Integrating Formose Reaction and Ribonucleotide Production
In a groundbreaking study, researchers have attempted to overcome the challenges posed by the formose reaction by introducing a simple molecule called cyanamide.
This addition enables some of the reaction products to be diverted towards the production of ribonucleotides. While the yield of ribonucleotide building blocks is not extensive, the ones produced exhibit increased stability and reduced susceptibility to degradation.
A Paradigm Shift in Chemical Thinking
This study marks a departure from the conventional reductionist approach in chemistry, where simplicity is favored to maximize product quantity and purity.
Researchers want to learn more about how different chemical pathways interact with each other by combining the formose reaction with ribonucleotide production. This will give them a more complete picture of the complicated processes that led to the start of life.
Industrial Implications of Autocatalysis
Beyond unraveling the mysteries of life’s origins, autocatalysis holds significant industrial potential. Adding cyanamide to the formose reaction not only makes it easier to make ribonucleotides but also makes other useful chemicals, like 2-amino oxazole. This compound has applications in chemical research and pharmaceutical production.
Optimizing Autocatalytic Processes
- The research team is currently focused on optimizing the procedure, aiming to manipulate autocatalytic reactions for industrial applications.
- For instance, the production of 2-amino oxazole, a compound vital to pharmaceuticals, could become more cost-effective by utilizing the formose reaction.
- This optimization could revolutionize common chemical reactions, making them more efficient and pharmaceutical products more accessible.
Conclusion
While the study of autocatalysis and the formose reaction may not recreate the grandiosity of life’s creation, it represents a significant step forward in understanding the intricate processes that might have led to the formation of RNA on early Earth.
The integration of autocatalytic reactions into established chemical pathways challenges traditional thinking in chemistry and opens doors to both scientific and industrial advancements.
As researchers continue to explore the complex tapestry of prebiotic chemistry, the quest for the dawn of life unveils new layers of understanding, bringing us closer to unraveling one of the greatest mysteries in the history of our planet.


 TSPSC Group 1 Question Paper 2024, Downl...
TSPSC Group 1 Question Paper 2024, Downl...
 TSPSC Group 1 Answer key 2024 Out, Downl...
TSPSC Group 1 Answer key 2024 Out, Downl...
 UPSC Prelims 2024 Question Paper, Downlo...
UPSC Prelims 2024 Question Paper, Downlo...
