The reactivity series, also known as the activity series, is a list of metals and elements organized in order of their reactivity from the most reactive to the least reactive. This series helps to predict the behavior of metals when they come into contact with substances like acids or water.
Reactivity Series
Here is the reactivity series in decreasing order of reactivity:
Potassium (K): Highly reactive metal, reacts vigorously with water.
Sodium (Na): Reacts similarly to potassium, and also reacts with water to produce hydrogen gas and hydroxide ions.
Calcium (Ca): Reacts with water, albeit less vigorously than potassium and sodium.
Magnesium (Mg): Reacts with steam and acids, but its reaction with water is slow.
Aluminum (Al): Reacts with strong acids, but it forms a protective oxide layer on its surface, preventing further reaction.
Zinc (Zn): Reacts with acids, releasing hydrogen gas.
Iron (Fe): Less reactive than zinc, reacts with acids, but its reaction rate is slower.
Tin (Sn): Reacts with acids but slowly, and with a tendency to form a protective oxide layer.
Lead (Pb): Reacts slowly with acids, mainly due to its oxide layer.
Hydrogen (H): Not a metal but included in the series; can be displaced by metals higher in the series from acids.
Copper (Cu): Does not readily react with dilute acids.
Silver (Ag): Generally does not react with acids.
Gold (Au): Highly unreactive, does not react with acids.
The reactivity series helps us understand and predict various chemical reactions, particularly in terms of displacement reactions. In a displacement reaction, a more reactive metal can displace a less reactive metal from its compound. This series is also useful in explaining why certain metals are used for specific applications, such as corrosion resistance or electrical conductivity.
What is the Reactivity Series?
The reactivity series of metals, also known as the activity series, refers to the arrangement of metals in the descending order of their reactivities.
Reactivity Series Class 10
Chemical reagents react differently with different metals. Metals that lose electrons more readily produce positive ions. In salt solution, a more reactive metal displaces a less reactive metal. These are known as displacement responses. Consider how zinc is displaced from copper sulphate when a small amount of zinc is immersed in a copper sulphate solution. Copper sulphate’s blue colour disappears, leaving a colourless zinc sulphate solution. Zinc is thus more reactive than copper.
Aluminium is more reactive than zinc, iron, and copper because it has the ability to displace them from their salt solutions. Zinc is more reactive than iron and copper because it has the capacity to displace them from their salt solutions. When compared to Cu, iron has the ability to displace copper from its salt solution, making it more reactive. The following equations shows the displacement of a less reactive element by a more reactive one:
2Al(s) + 3ZnSO4(aq) –→ Al2(SO4)3 + 3Zn(s)
2Al(s) + 3FeSO4(aq) –→ Al2(SO4)3 + 3Fe(s)
2Al(s) + 3CuSO4(aq) –→ Al2(SO4)3 + 3Cu(s)
Fe(s) + CuSO4(aq) –→ FeSO4 (aq) + Cu(s)
Zn(s) + CuSO4(aq) –→ ZnSO4 (aq) + Cu(s)
Zn(s) + FeSO4(aq) –→ ZnSO4 (aq) + Fe(s)
Reactivity Series of Metals for Class 10
Metals are arranged in descending order of their reactivities in the reactivity series, which is also known as the activity series. The reactivity series data can be used to determine if a metal can displace another in a single displacement reaction. A metal higher up in the reactivity series displaces all other metals in a compound below it in a displacement reaction. Zinc, which is more active than copper, for example, replaces copper sulphate in solution to generate zinc sulphate and free copper.
The ability of a metal to lose electrons in solution and create positive ions determines its activity. The more easily a metal loses electrons, the more active it is and the higher up in the reactivity series it is. The most active metal, cesium, is at the top of the reactivity scale, while the least active metal, platinum, is at the bottom. Following is the list of metals in order of their reactivity strength:
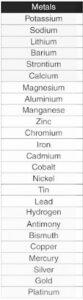
Reactivity Series of Non Metals for Class 10
Nonmetals, like metals, can be arranged according to their reactivity. A more active nonmetal displaces a less active nonmetal from a combination during displacement reactions. The ability of a nonmetal to acquire electrons in the solution state and produce positive ions determines its activity. The more easily a nonmetal obtains electrons, the more active it is and the farther along the reactivity series it is. Following is the list of non metals in the order of their reactivity strength:

Metal Reactivity Series in Table
Reactivity Series is given below. check here.
| Reactivity Series of Metals | Ions Formed |
| Caesium | Cs+ |
| Francium | Fr+ |
| Rubidium | Rb+ |
| Potassium | K+ |
| Sodium | Na+ |
| Lithium | Li+ |
| Barium | Ba2+ |
| Radium | Ra2+ |
| Strontium | Sr2+ |
| Calcium | Ca2+ |
| Magnesium | Mg2+ |
| Beryllium | Be2+ |
| Aluminium | Al3+ |
| Titanium | Ti4+ |
| Manganese | Mn2+ |
| Zinc | Zn2+ |
| Chromium | Cr3+ |
| Iron | Fe3+ |
| Cadmium | Cd2+ |
| Cobalt | Co2+ |
| Nickel | Ni2+ |
| Tin | Sn2+ |
| Lead | Pb2+ |
| Hydrogen | H+ (Non-Metal, Reference for Comparison) |
| Antimony | Sb3+ |
| Bismuth | Bi3+ |
| Copper | Cu2+ |
| Tungsten | W3+ |
| Mercury | Hg2+ |
| Silver | Ag+ |
| Platinum | Pt4+ |
| Gold | Au3+ |
Non Metal Reactivity Series
non-metals can still be compared based on their electronegativity or reactivity in specific reactions. Here is a list of some non-metals ordered by increasing electronegativity, which can give you a general idea of their reactivity with other elements:
- Hydrogen (H)
- Helium (He)
- Neon (Ne)
- Argon (Ar)
- Krypton (Kr)
- Xenon (Xe)
- Radon (Rn)
- Fluorine (F)
- Oxygen (O)
- Nitrogen (N)
- Chlorine (Cl)
- Bromine (Br)
- Iodine (I)
- Astatine (At)
Uses of Reactivity Series
The reactivity series, which ranks metals and elements based on their reactivity, has several practical applications across various fields. Here are some of the key uses of the reactivity series:
Predicting Chemical Reactions: The reactivity series helps predict how different metals will react with acids, water, and other substances. This information is particularly important in understanding and predicting displacement reactions, where a more reactive metal can displace a less reactive metal from its compound.
Selection of Metals for Different Applications: The reactivity series plays a role in selecting metals for specific applications. For example:
-
-
- Metals at the top of the series, such as potassium and sodium, are highly reactive and can be used as reducing agents in various chemical processes.
- Less reactive metals like gold and platinum are used in jewelry and electronic components due to their resistance to corrosion.
-
- Corrosion Prevention: Knowledge of the reactivity series helps engineers and scientists choose appropriate materials to prevent corrosion. For example, metals like aluminum and zinc are often used as sacrificial anodes in corrosion protection systems, where they corrode preferentially to protect more valuable metals from corrosion.
- Extraction of Metals from Ores: The reactivity series aids in understanding the extraction of metals from their ores. Metals high in the reactivity series, such as sodium and potassium, are extracted using electrolysis, while metals lower in the series, like iron and copper, can be extracted using more conventional methods like reduction with carbon.
- Understanding Redox Reactions: The reactivity series is closely related to redox (reduction-oxidation) reactions. It helps explain the transfer of electrons in reactions involving metals and their compounds.
- Education and Science Communication: The reactivity series is often taught in schools and serves as an introductory concept in chemistry education. It helps students understand basic concepts of chemical reactions and the behavior of different metals.
- Environmental and Industrial Chemistry: In environmental chemistry, the reactivity series is important for understanding interactions between metals and pollutants in soil and water. In industrial chemistry, it is relevant for processes involving metal compounds, such as in catalysis.
- Quality Control in Metallurgical Processes: The reactivity series is considered in metallurgical processes to control reactions and ensure desired outcomes, such as the reduction of metal oxides during the extraction process.
In summary, the reactivity series is a fundamental concept in chemistry that has practical applications in various scientific, industrial, and technological contexts. It guides decision-making, material selection, and process design across a range of disciplines.
Reactivity Series in Hindi
रासायनिक अभिकर्मक विभिन्न धातुओं के साथ अलग-अलग प्रतिक्रिया करते हैं। धातुएँ जो इलेक्ट्रॉनों को अधिक आसानी से खो देती हैं वे सकारात्मक आयन उत्पन्न करती हैं। नमक के घोल में, अधिक प्रतिक्रियाशील धातु कम प्रतिक्रियाशील धातु को विस्थापित करती है। इन्हें विस्थापन प्रतिक्रिया के रूप में जाना जाता है। गौर कीजिए कि कॉपर सल्फेट के घोल में जिंक की थोड़ी मात्रा डालने पर जिंक कॉपर सल्फेट से कैसे विस्थापित होता है। कॉपर सल्फेट का नीला रंग गायब हो जाता है, जिससे जिंक सल्फेट का रंगहीन घोल निकल जाता है। इस प्रकार जस्ता तांबे की तुलना में अधिक प्रतिक्रियाशील है।
जिंक, आयरन और कॉपर की तुलना में एल्युमिनियम अधिक प्रतिक्रियाशील होता है क्योंकि इसमें नमक के घोल से उन्हें विस्थापित करने की क्षमता होती है। जस्ता लोहे और तांबे की तुलना में अधिक प्रतिक्रियाशील है क्योंकि इसमें नमक के घोल से उन्हें विस्थापित करने की क्षमता है। Cu की तुलना में, लोहे में तांबे को अपने नमक के घोल से विस्थापित करने की क्षमता होती है, जिससे यह अधिक प्रतिक्रियाशील हो जाता है। निम्नलिखित समीकरण एक कम प्रतिक्रियाशील तत्व के अधिक प्रतिक्रियाशील एक द्वारा विस्थापन को दर्शाता है:
2Al(s) + 3ZnSO4(aq) –→ Al2(SO4)3 + 3Zn(s)
2Al(s) + 3FeSO4(aq) –→ Al2(SO4)3 + 3Fe(s)
2Al(s) + 3CuSO4(aq) –→ Al2(SO4)3 + 3Cu(s)
Fe(s) + CuSO4(aq) –→ FeSO4 (aq) + Cu(s)
Zn(s) + CuSO4(aq) –→ ZnSO4 (aq) + Cu(s)
Zn(s) + FeSO4(aq) –→ ZnSO4 (aq) + Fe(s)
धातुओं की प्रतिक्रियाशीलता श्रृंखला
धातुओं को उनकी प्रतिक्रियाशीलता के अवरोही क्रम में प्रतिक्रियाशीलता श्रृंखला में व्यवस्थित किया जाता है, जिसे गतिविधि श्रृंखला के रूप में भी जाना जाता है। प्रतिक्रियाशीलता श्रृंखला डेटा का उपयोग यह निर्धारित करने के लिए किया जा सकता है कि क्या एक धातु एक विस्थापन प्रतिक्रिया में दूसरे को विस्थापित कर सकती है। अभिक्रियाशीलता श्रेणी में ऊपर की ओर एक धातु विस्थापन अभिक्रिया में अपने नीचे के यौगिक में अन्य सभी धातुओं को विस्थापित कर देती है। जस्ता, जो तांबे से अधिक सक्रिय है, उदाहरण के लिए, जिंक सल्फेट और मुक्त तांबा उत्पन्न करने के लिए समाधान में कॉपर सल्फेट की जगह लेता है।
किसी धातु की विलयन में इलेक्ट्रॉनों को खोने और सकारात्मक आयन बनाने की क्षमता उसकी गतिविधि को निर्धारित करती है। एक धातु जितनी आसानी से इलेक्ट्रॉनों को खो देती है, वह उतनी ही अधिक सक्रिय होती है और प्रतिक्रियाशीलता श्रृंखला में उतनी ही ऊपर होती है। सबसे सक्रिय धातु, सीज़ियम, प्रतिक्रियाशीलता पैमाने के शीर्ष पर है, जबकि सबसे कम सक्रिय धातु, प्लैटिनम, सबसे नीचे है।
गैर धातुओं की प्रतिक्रियाशीलता श्रृंखला
धातुओं की तरह अधातुओं को उनकी अभिक्रियाशीलता के अनुसार व्यवस्थित किया जा सकता है। एक अधिक सक्रिय अधातु विस्थापन प्रतिक्रियाओं के दौरान संयोजन से कम सक्रिय अधातु को विस्थापित करती है। समाधान अवस्था में इलेक्ट्रॉनों को प्राप्त करने और सकारात्मक आयनों का उत्पादन करने के लिए एक अधातु की क्षमता इसकी गतिविधि को निर्धारित करती है। एक अधातु जितनी आसानी से इलेक्ट्रॉन प्राप्त करती है, वह उतनी ही अधिक सक्रिय होती है और प्रतिक्रियाशीलता श्रृंखला के साथ उतनी ही दूर होती है।
Related Post:
- Letter To Editor- Format, With Sample Questions For Class 10
- a3 B3 Formula- Proof, Solution And Examples
- Scientific Name Of Goat In India- Pronunciation
- Cube Formula For Area, Volume In Maths
- Father Of Economics- Adam Smith
- Who Was The Last Viceroy Of India?
- Planck’s Constant- Value, Definition, Unit, Symbol, Formula
- Bohr Atomic Model- Diagram, Formula, Postulates And Limitations
- Median Formula For Even, Odd, And Grouped Data
- The Largest Country In The World By Area & Population








 BTEUP Result 2025 Out at bteup.ac.in, Do...
BTEUP Result 2025 Out at bteup.ac.in, Do...
 SGBAU Result 2025 Out, Check Summer Seme...
SGBAU Result 2025 Out, Check Summer Seme...
 How To Prepare for CUET Accountancy Exam...
How To Prepare for CUET Accountancy Exam...









