Mass of Electron
Mass of Electron, Proton, Neutron: The mass of a stationary electron is referred to as the electron mass. It is also referred to as the electron’s invariant mass and is one of physics’s fundamental constants. It weighs approximately 9.1091031 kilograms, or 5.486104 daltons. An electron’s mass equals approximately 8.1871014 joules, or 0.5110 MeV. In comparison to a proton or a neutron, an electron is thought to be nearly massless. The mass of an electron is not considered when calculating an atom’s mass number. The electron, with a negative charge of 1.602176634 1019 coulomb, is the lightest stable subatomic particle known
Mass of Electron in KG and Mev
Electron weighs around 9.1091031 kilogrammes or 5.486104 daltons. The mass of an electron corresponds to an energy of around 8.1871014 joules, or about 0.5110 MeV.
Mass of Electron in AMU
Mass of electron is amu.
Read More About: Sin 0 Degrees Value
Mass of Electron is Equal to
J.J. Thomson, an English physicist, discovered the electron in 1897. He found it while investigating cathode rays, and his discovery of electrons revolutionised our understanding of atomic structure. Under normal circumstances, electrons are attracted to positively charged nuclei of atoms by the attraction of opposite electric charges, and the number of electrons in a neutral atom is equal to the number of positive charges on the nucleus. However, each atom can be negatively or positively charged depending on whether it has more or fewer electrons than positive charges. These charged atoms are also referred to as ions. In the type of matter known as plasma, some electrons exist in a free state with ions. Electrons circulate about the nucleus in an organised array of orbitals within each atom. The electrons’ attraction to the nucleus overcomes the electrons’ repulsion, which would otherwise force them to fly apart. These orbitals are structured in concentric shells with an increasing number of subshells that radiate outward from the nucleus, with the electrons in the orbitals nearest to the nucleus being held the most securely. The outermost orbitals, on the other hand, are sheltered by adjacent electrons and are the least tightly held by the nucleus. The electrons in this arrangement produce a diffuse cloud of negative charge as they travel about. It also takes up virtually the whole volume of the atom. The electronic configuration of an atom refers to the specific structural arrangement of electrons within an atom, which includes not only the size of an individual atom but also the chemical composition of the atom.
Electron Charge
The natural unit of electric charge is the electron charge, which is a fundamental scientific constant. The charge of an electron is 1.602176634 1019 coulomb. This value, or a whole-number multiple of it, is shared by all freely existent charged subatomic particles identified thus far. Charges of 1/3 or 2/3 of this magnitude are found in quarks, which are constantly bonded within larger subatomic particles like protons and neutrons.
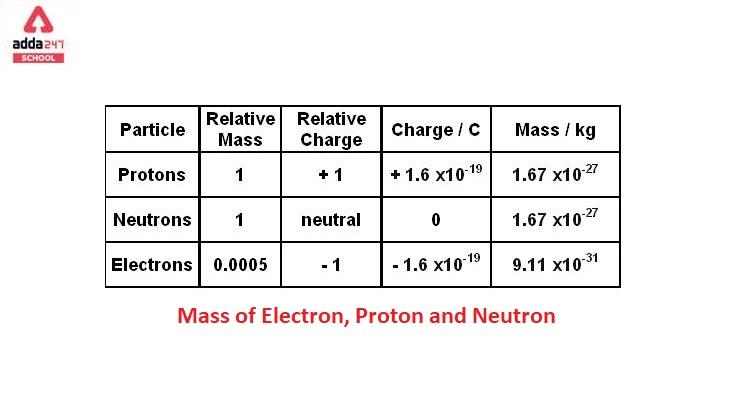
Mass of Electron, Proton and Neutron
Mass of Electron, Proton and Neutron is given below.
Mass of Electron
*The proton is a subatomic particle that is stable. It has a rest mass of 1.67262 1027 kg and a positive charge equivalent to a unit of electron charge. The mass of an electron is 1,836 times the rest mass of 1.67262 1027 kg.
All atomic nuclei are made up of protons and neutrons, which are electrically neutral particles. The number of protons in each nucleus of a chemical element is the same, and this quantity determines the element’s atomic number. It also establishes the element’s place in the periodic table. The atom is electrically neutral when the number of protons in the nucleus equals the number of electrons orbiting the nucleus.
Charge on Electron
The charge on an electron is approximately -1.602 x 10^-19 coulombs (C). This value is often rounded to -1.6 x 10^-19 C for simplicity in calculations. Electrons are negatively charged subatomic particles that orbit the nucleus of an atom, and their charge is equal in magnitude but opposite in sign to the charge of a proton, which has a charge of approximately +1.602 x 10^-19 C. This fundamental charge is a fundamental constant in physics and plays a crucial role in the behavior of matter at the atomic and subatomic levels.
Mass of Proton
Moreover, the charge of a proton is 1.6022 x 10-19 coulomb.
*Protons and electrons were thought to be the two fundamental particles until 1930, when physicist James Chadwick discovered neutrons in 1932.
He conducted an experiment in which he discovered that blasting beryllium with alpha particles resulted in the emission of neutral radiations.
Because protons are charged particles that can be deflected on a curving path towards the negative plate, he determined that they are not protons using conservation of energy and momentum. It denotes the presence of something without a charge.
Charge of Proton
The charge of a proton is approximately +1 elementary charge, which is equal to approximately 1.602 x 10^-19 coulombs. In atomic and particle physics, the elementary charge is often denoted as “e,” and it serves as a fundamental unit of electric charge. Protons are one of the fundamental particles found in the nucleus of an atom and carry a positive electric charge, which is equal in magnitude but opposite in sign to the charge of an electron, which is -1e.
Mass of Neutron
It’s the neutron, of course.
Neutrons are hence non-charged subatomic particles.
A free neutron has a mass of 1.6749286 x 1027 kg, or 939,565,346 eV/c2.
The units of mass and energy are interchangeable in ordinary particle physics.
Charge of Neutron
The neutron is a subatomic particle that is electrically neutral, meaning it has no net electric charge. It is often symbolized as “n” and is found in the nucleus of atoms along with protons, which are positively charged. Protons have a charge of +1, while electrons, which orbit the nucleus, have a charge of -1. Neutrons and protons are collectively referred to as nucleons and play a crucial role in determining the properties and stability of atomic nuclei.
Related Post: Capital Of Gujarat








 BTEUP Result 2025 Out at bteup.ac.in, Do...
BTEUP Result 2025 Out at bteup.ac.in, Do...
 SGBAU Result 2025 Out, Check Summer Seme...
SGBAU Result 2025 Out, Check Summer Seme...
 How To Prepare for CUET Accountancy Exam...
How To Prepare for CUET Accountancy Exam...









