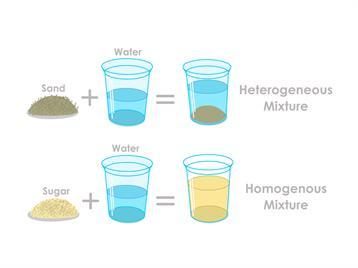
A homogeneous mixture has a uniform composition throughout, appearing the same in solid, liquid, or gas form. Examples include water, air, alloys, and saltwater. These are often mistaken for pure substances. In contrast, a heterogeneous mixture has unevenly distributed components that can be separated by physical methods like filtration and sedimentation.
What is Homogenous Mixture?
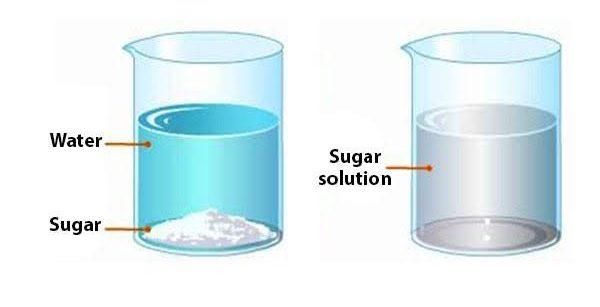
A homogenous mixture is one that has a consistent composition and qualities throughout. The salt water is homogenous mixture because the salt that has been dispersed is evenly distributed across the entire salt water sample. The amount of salt in the saltwater varies from sample to sample. Because the dissolved substance is present in the same amount throughout the solution, all solutions are termed homogenous. They can be separated physically. Because there are no chemical changes, the specific qualities of the components stay unchanged. Every component in a mixture has its own identity. There is no change in energy during the production of a mixture, and the boiling and melting points of the mixture are determined by the properties of the elements.
What is Heterogeneous Mixture?
A heterogeneous mixture is a type of mixture in which the components are not uniformly distributed throughout the mixture. In other words, the different substances that make up the mixture can be distinguished from each other by visual observation or by using simple laboratory techniques. Each component retains its own properties and does not blend smoothly with the others.
Heterogeneous Mixture Definition
In chemistry, a heterogeneous mixture is one that contains either one or both of numerous states of matter or hydrophilic and hydrophobic compounds. An example of the latter would be a mixture of water, octane, and silicone grease. Solids, liquids, and gases that are heterogeneous can be made homogeneous by melting, stirring, or by allowing the molecules to diffuse over time. For instance, if dye is added to water, the resulting solution will initially be heterogeneous but will eventually become homogeneous. Heterogeneous materials can eventually become homogeneous thanks to entropy.
Homogeneous Mixture Examples
A mixture is characterized as a physically combined combination of two or more substances. Almost everything we see around us is a combination of one or more substances. For example, food is a mixture of numerous substances, air is a mixture of gases, and the gasoline we use in automobiles is also a mixture of Hydrocarbons. A mixture is another term for impure things. The following are some examples of the mixture:
- Air: Air is a homogeneous mixture.It is a mixture of gases such as oxygen, carbon dioxide, nitrogen, argon, neon, and others. The Earth’s atmosphere, on the other hand, is a heterogeneous combination. The clouds are proof that the composition is not consistent.
- Alloys are created by combining two or more metals. They are often homogenous mixes. Brass, bronze, steel, and sterling silver are among examples. Alloys can have many phases at times.
- Seawater is a combination of different salts and water.
- Sugar water
- Crude Oil: It is a mixture of Hydrocarbons.
- Rainwater
- Vinegar
- Cup of Coffee
- Dishwashing detergent
- Steel
- Cologne
- Mouthwash
Heterogeneous Mixture Example
A mixture of two or more chemicals is referred to as a heterogeneous mixture. Examples include conglomerate rocks, water and oil, salads, trail mix, and mixes of sand and water or sand with iron filings (not cement).
A heterogeneous mixture is a combination of different substances that are not uniformly distributed and can be distinguished from one another. Here’s an example of a heterogeneous mixture:
Trail Mix: Trail mix is a classic example of a heterogeneous mixture. It’s typically made up of various components such as nuts, raisins, chocolate chips, dried fruits, and sometimes even pretzels or seeds. When you look at a handful of trail mix, you can easily see and distinguish the different components. They don’t blend together to form a uniform mixture; instead, they maintain their individual identities within the mixture. This non-uniform distribution of components is what makes trail mix a heterogeneous mixture.
Examples of Heterogeneous Mixture
Examples of heterogeneous mixtures include:
- Salad: A salad is a classic example of a heterogeneous mixture. It consists of various ingredients like lettuce, tomatoes, cucumbers, and dressing, which can be easily identified and separated.
- Granite: Granite is a natural rock that contains different minerals like quartz, feldspar, and mica. These minerals are distributed unevenly within the rock, making it a heterogeneous mixture.
- Sand and Water: If you mix sand and water, the sand particles will not dissolve and will settle at the bottom over time, forming a distinct layer. This demonstrates the heterogeneous nature of the mixture.
- Trail Mix: Trail mix often contains a variety of components such as nuts, dried fruits, chocolate chips, and seeds. These components can be seen separately and are not evenly distributed.
- Oil and Vinegar Dressing: Oil and vinegar do not mix completely, forming separate layers in a salad dressing. This is due to their immiscibility and is a characteristic of a heterogeneous mixture.
10 Examples of Heterogeneous Mixture
ten examples of heterogeneous mixtures are given below.
Salad – Different ingredients like lettuce, tomatoes, cucumbers, and dressing are visible and separable.
Granite – A rock composed of different minerals, such as quartz, feldspar, and mica, that can be seen with the naked eye.
Sand and Salt – A mixture where grains of sand and salt can be distinguished and separated.
Oil and Water – When mixed, they form two distinct layers due to their different densities.
Trail Mix – A combination of nuts, dried fruits, and chocolate pieces, where each component is easily identifiable.
Concrete – A mixture of cement, water, sand, and gravel, where the individual components can still be recognized.
Fruit Cocktail – A mix of different fruits that retain their individual textures and colors.
Pulpy Juice – Juice with pulp from fruits, where the solid parts can be seen and separated from the liquid.
Cereal and Milk – Cereal pieces float in milk, maintaining distinct textures and flavors.
Mud – A mixture of water and soil, where the soil particles can be seen suspended in the liquid.
Homogenous Mixture
Heterogeneous mixture are the varieties of mixtures where the ingredients are evenly dispersed throughout. To put it another way, “they are consistent throughout.” In a homogeneous mixture, there is only one phase of matter that can be observed. Important details about such mixes include the distribution of particles is uniform, we cannot assess a homogeneous mixture based solely on appearance, solutions are another name for homogeneous mixes, and consistent composition.
10 Examples of Homogeneous Mixture
Check 10 examples of homogeneous mixtures, which are given below
Saltwater – Salt dissolved in water.
Air – A mixture of gases like nitrogen, oxygen, and small amounts of other gases.
Vinegar – A mixture of acetic acid and water.
Steel – An alloy of iron and carbon (and sometimes other elements).
Alcoholic beverages – Like vodka or whiskey, where ethanol is mixed with water and other substances.
Sugar water – Sugar fully dissolved in water.
Brass – An alloy of copper and zinc.
Coffee – Brewed coffee is a mixture of water and coffee solubles.
Milk – Milk is a colloidal suspension that appears uniform at the macro level.
Gasoline – A mixture of hydrocarbons and other compounds that blend to form a single phase.
Acetone Formula, Structure, Name, Uses, Reactions in Chemistry
Difference Between Homogeneous and Heterogeneous Mixture
Following table depicts the difference between the homogenous mixture and heterogeneous mixture.
Homogeneous mixture |
Heterogeneous mixture |
| Homogeneous mixture has a uniform composition | Heterogeneous mixture has a non-uniform composition |
| Homogeneous mixture has only one phase | There are two or more phases in Heterogeneous mixture |
| Homogeneous mixture can’t be separated out physically | Heterogeneous mixture can be separated out physically |
| ‘homo’ refers to same | ‘hetero’ refers to different |
| Eg. Mixture of salt and water, Mixture of water and sugar. | Eg. Mixture of Sand and water, Mixture of oil and water. |
Modern Periodic Table of Elements with Name & Atomic mass
Important Homogeneous & Heterogenous Mixture Classification
Homogeneous and Heterogenous mixtures can be further categorized as follows:
Suspensions
A suspension is a mixture of two or more different substances. The particles are suspended in bulk throughout the fluid.
- The particles are larger than 5 x 10-7 m in size, which means they can be seen with the naked eye.
- All of the components are totally combined in this form of mixture, and all of the particles can be seen under a microscope.
- The particles tend to settle (or float) over time and must be mixed again to restore the suspended condition.
- The particles are not filtered by filter paper. Filtration can therefore separate a suspension. Because of its enormous particle size, it scatters the light beam travelling through it.
Example – Paint mucky water A flour and water combination, Water and flour Slaked lime for whitewashing, and so on.
Solutions
A solution is a homogenous mixture of two or more constituents. Solute particles in the solutions are exceedingly tiny.
- The particles have a size of less than 2 x 10-9 m. They are so small that you can’t tell the difference between the solute (what’s being dissolved) and the solvent (what dissolves the solute).
- example, salt water, sugar water, soda water dissolves in water, and so on. The solute and the solvent are the two main components of a solution.
- Solvent: A solvent is the component in a solution that dissolves the other component. The solvent is the most important component of a solution. For instance, in sugar water. Sugar is a solute, whereas water is a solvent.
- Solute: A solute is a component of a solution that melts in a solvent. In the given solution, the solute is the smaller component. In sugar water, sugar acts as a solute, however, iodine is the solute in a solution of iodine in alcohol known as “tincture of iodine.”
Colloids
colloids are mixtures in which microscopically distributed insoluble elements of one substance remain suspended in another.
- Because of their small sizes, intermolecular interactions are strong enough to overcome the tendency of particles to settle or float.
- The combination in which suspended particles do not sink at the bottom but are equally disseminated throughout another substance.
- Colloids have particle sizes ranging from 2 x 10-9 m to 5 x 10-7 m.
Types of Colloids
Colloidal Solutions are classified as sol, emulsion, foam, aerosol, and gel.
- Sol is a colloidal system with a solid dispersed phase and a liquid dispersion medium. For example, blood, ink, paint, mud, and so on.
- Foam is a colloidal system with a gas-dispersed phase and a solid or liquid dispersion medium. Whipped cream, bubble bath, fire retardant, and so on.
- An emulsion is a colloidal system with a liquid dispersed phase and another liquid as the dispersion medium. For example, milk, salad dressing, and freshly brewed coffee.
- An aerosol is a colloidal system with a solid or liquid particle as the dispersed phase and a gas as the dispersion medium. For example, hairspray, perfume, mist, fog, and so on.
- The gel is a colloidal system with a solid dispersed phase and a liquid dispersion medium. For example, toothpaste, jam, cheese, rubber, gelatin, and so forth.
Heterogeneous Mixture Meaning in Hindi
रसायन विज्ञान में, एक विषम मिश्रण वह होता है जिसमें या तो एक या दोनों पदार्थ या हाइड्रोफिलिक और हाइड्रोफोबिक यौगिकों के कई राज्य होते हैं। उत्तरार्द्ध का एक उदाहरण पानी, ऑक्टेन और सिलिकॉन ग्रीस का मिश्रण होगा। ठोस, तरल और गैस जो विषम हैं, उन्हें पिघलाकर, हिलाकर या समय के साथ अणुओं को फैलाने की अनुमति देकर सजातीय बनाया जा सकता है। उदाहरण के लिए, यदि डाई को पानी में मिलाया जाता है, तो परिणामी घोल शुरू में विषम होगा लेकिन अंततः सजातीय हो जाएगा। एन्ट्रॉपी के कारण विषम सामग्री अंततः सजातीय बन सकती है।
Heterogeneous Mixture Example in Hindi
दो या दो से अधिक रसायनों के मिश्रण को विषमांगी मिश्रण कहते हैं। उदाहरणों में सम्मिलित चट्टानें, पानी और तेल, सलाद, पगडंडी का मिश्रण, और रेत और पानी का मिश्रण या लोहे के बुरादे वाली रेत (सीमेंट नहीं) शामिल हैं।
इस तरह के संयोजन में इसके सभी घटक अच्छी तरह से संयुक्त होते हैं, और इसके सभी कण सूक्ष्मदर्शी से दिखाई देते हैं। भागों को पहचानना आसान है, और बिना सहायता प्राप्त आंखों से एक से अधिक चरण दिखाई दे सकते हैं। इस प्रकार के मिश्रण के बारे में महत्वपूर्ण जानकारी हम यह बता सकते हैं कि क्या कोई संयोजन केवल देखने से ही विषम है, कणों का वितरण एक समान नहीं है, और रचना एक समान नहीं है।
Also Read:
- Parenchyma- Cells, Tissue, Meaning, Function, And Diagram
- What Is Hybridization?- Sp3, Sp2, Examples And Formula
- What Is A Stethoscope?- Its Parts, Uses, & Diagram
- Parallel Axis Theorem, Proof, Definition, Formula, Examples
- Oscillatory Motion, Meaning, Definition, Example, Diagram
- Human Eye Definition, Diagram, Structure
- What Is The Unit Of Current, Resistance And Voltage?
- All About Father Of Genetics- Gregor Mendel









 DTE Maharashtra Polytechnic Merit List 2...
DTE Maharashtra Polytechnic Merit List 2...
 JEECUP Round 2 Seat Allotment Result 202...
JEECUP Round 2 Seat Allotment Result 202...
 YCMOU Result 2025 Out @ycmou.digitaluniv...
YCMOU Result 2025 Out @ycmou.digitaluniv...









