Table of Contents
Difference Between Acid and Base
Difference between acid and base is discussed in this article. Acid and base are the basics of chemistry that we learn in lower grades. Acid and base form the pillars of chemistry. Acids are in the form of lactic or citrus acid which is mostly present in citrus fruits like lemon or orange. Bases are in powder form mostly like bleaching powder, ammonia, etc. The acid-base helps in differentiating several compounds in chemistry.
Acid and Base Difference
What is Acid?
There are several different definitions of acids according to many scientists. According to Arrhenius, any substance when dissolved in water increases the concentration of hydrogen ions or H+ ions are acid.
Bronsted-Lowry defined Acid as a proton donor. This means when a compound donates the proton easily and as soon as possible, it is considered a strong acid.
Another definition is given by Lewis, that is, the compound that accepts a pair of electrons. That means any compound that is electron deficient can act as an acid.
Types of Acids
- Strong acids- the acids that can dissociate ions easily and completely in water are called strong acids.
Example- HCl, H2SO4, HNO3, etc.
- Weak acids- The acids that can partly dissociate ions in water are called weak acids.
What is Base?
Similar to acid, these three scientists have a different definition for the base too.
According to Arrhenius, any substance, when dissolved in water increases the concentration of OH– ions are base.
The Bronsted-Lowry base theory states that any substances that accept a proton are base. The faster it accepts a proton the stronger the basicity.
Lewis’s definition of base is that any substance that accepts a pair of the electron is base.
Types of bases-
- Strong Base
- Weak Base
- Neutral Base
State differences between acids and bases Class 7
Acids and bases are two fundamental categories of substances in chemistry, and they differ in several key ways:
- Definition:
- Acid: Acids are substances that can donate a proton (H+) or accept an electron pair in a chemical reaction. In the Brønsted-Lowry definition, acids are proton donors.
- Base: Bases are substances that can accept a proton (H+) or donate an electron pair in a chemical reaction. In the Brønsted-Lowry definition, bases are proton acceptors.
- pH:
- Acids have a pH less than 7 on the pH scale. The lower the pH, the stronger the acid.
- Bases have a pH greater than 7 on the pH scale. The higher the pH, the stronger the base.
- Taste and Feel:
- Acids often taste sour. For example, lemon juice is acidic.
- Bases often taste bitter and feel slippery to the touch. For example, soap is a base.
- Chemical Properties:
- Acids react with metals to produce hydrogen gas.
- Bases can react with fats and oils (in a process called saponification) to form soap.
- Examples:
- Common examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (found in vinegar).
- Common examples of bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).
- Neutralization:
- When an acid reacts with a base, they neutralize each other, forming water and a salt. This reaction is called neutralization.
- Indicator Dyes:
- Acids turn blue litmus paper red.
- Bases turn red litmus paper blue.
- Strength:
- Acids and bases can be classified as strong or weak based on their ability to ionize in solution. Strong acids and bases ionize completely, while weak ones only partially ionize.
- Chemical Formulas:
- Acids often have the hydrogen ion (H+) in their chemical formulas, such as HCl for hydrochloric acid.
- Bases often have hydroxide ions (OH-) in their chemical formulas, such as NaOH for sodium hydroxide.
It’s important to note that the Brønsted-Lowry definition of acids and bases is just one of several definitions in chemistry. The Lewis definition, for example, focuses on electron pair donors and acceptors and is more general, encompassing a wider range of chemical reactions involving acids and bases.
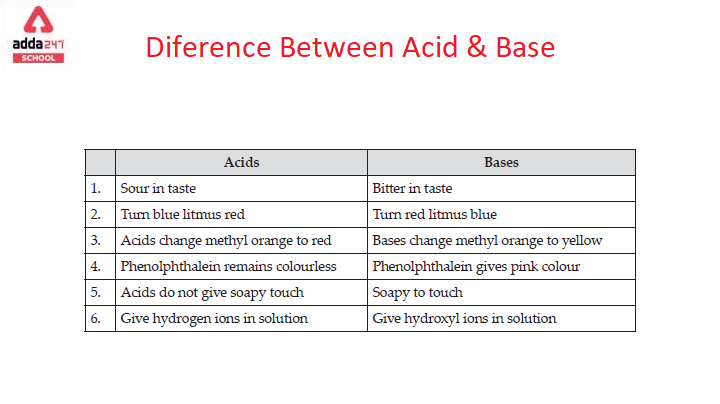
Difference Between Acids and Bases
difference between acids and bases are given below in the table.
| Basis | Acid | Base |
| Proton | Acid donates proton when dissolved in water | Base accepts proton when dissolved in water |
| Litmus paper test | Acid turns blue litmus into red litmus paper | The base turns red litmus into blue litmus paper |
| Salt formation | Acids form salt when it reacts with base in an aqueous solution | The base forms a salt when it reacts with the acid in an aqueous solution |
| Taste test | Taste Sour | Taste soapy |
Acids and bases have different properties with different chemical formulas. These are the basics of chemistry that help a lot throughout your journey in chemistry. Here we summed up the definition and differences between acid and base.
State difference between acid and base
Acids and bases are two fundamental categories of substances in chemistry, and they differ in several key ways, including their properties, chemical behavior, and effects on pH (a measure of acidity or alkalinity).
Other Related Posts –

 SOF Olympiad Exam Date 2024-25 Out, Chec...
SOF Olympiad Exam Date 2024-25 Out, Chec...
 NEET Revised Answer Key 2024 Out, Downlo...
NEET Revised Answer Key 2024 Out, Downlo...
 Karnataka PGCET Admit Card 2024 Out, Dow...
Karnataka PGCET Admit Card 2024 Out, Dow...












