Table of Contents
National Testing Agency will release the National Eligibility cum Entrance Test Syllabus 2025 on behalf of the NMC (National Medical Commission). The NEET Syllabus recently underwent major changes for the 2024 exam. Many chapters and topics were deleted and some new topics were added in the official syllabus. It is more likely that the NEET 2025 syllabus will be identical to the existing syllabus. Students can get the NEET syllabus 2025 in the article below along with the subject-wise syllabus PDF.
Check: NEET Revised Result 2024
NEET 2025 Syllabus
National Testing Agency will release the NEET Syllabus along with the NEET 2025 Notification. It is expected that the NEET 2025 syllabus will be released in December 2024 on the official website. Along with the NEET syllabus 2025, the NTA also releases the information brochure containing the NEET exam pattern and other important details. The NEET UG syllabus is formulated by the experts of the National Medical Commission, formerly the Medical Council of India. The formulated syllabus is then released by the NTA.
NEET Syllabus 2025
The NEET syllabus is essential to make your NEET 2025 Exam strategy. is certain to remain like its 2024 counterpart. It is so because the NEET syllabus was recently revised by the NMC two times for the NEET exam to be conducted in 2024. The syllabus was revised in line with the changes made in the board syllabus of different education boards due to the adoption of the NEP (National Education Policy).
NEET 2025 Syllabus NTA Official Website
The official syllabus for NEET 2025 has not yet been released, but you can expect it to be similar to previous years. The National Testing Agency (NTA) typically updates the syllabus for NEET on its official website https://exams.nta.ac.in/NEET/.
NTA NEET Syllabus 2025 for Physics
The Physics section has a weightage of 25% in the NEET exam. Due to its conceptual nature, students should start preparing the standard syllabus of the physics for NEET exam. The NEET Physics syllabus 2025 consists of chapters from class 11 and class 12 NCERT textbooks. The NEET 2025 updated syllabus for the Physics subject is given here. We will advise you to cover physics part properly to increase the marks in the UG exam.
NEET Syllabus Topics from Class 11
- UNIT I: Physical World and Measurement
- UNIT II: Kinematics
- UNIT III: Laws of Motion
- UNIT IV: Work, Energy, and Power
- UNIT V: Rotational Motion
- UNIT VI: Gravitation
- UNIT VII: Properties of Solids and Liquids
- UNIT VIII: Thermodynamics
- UNIT IX: Kinetic Theory of Gases
- UNIT X: Oscillations and Waves
NEET 2024 Syllabus Class 12 Topics
- UNIT I: Electrostatics
- UNIT II: Current Electricity
- UNIT III: Magnetic Effects of Current and Magnetism
- UNIT IV: Electromagnetic Induction and Alternating Currents
- UNIT V: Electromagnetic Waves
- UNIT VI: Optics
- UNIT VII: Dual Nature of Matter and Radiation
- UNIT VIII: Atoms and Nuclei
- UNIT IX: Electronic Devices
- Unit X: Experimental Skills
NEET Syllabus for Physics – Click to Download PDF
Physics Chapter-wise Weightage in NEET 2025
| Physics Chapter-wise Weightage in NEET 2025 | |
Class 11 |
|
| Chapter Name | No. of Questions |
| Work, Energy and Power | 1 |
| Gravitation | 2 |
| Circular Motion | 2 |
| Mechanical Properties of Fluids | 1 |
| Center of Mass and System of Particles | 1 |
| Oscillations | 2 |
| Kinetic Theory | 1 |
| Electromagnetic Waves | 2 |
| Motion in a Straight Line | 1 |
Class 12 |
|
| Moving Charges and Magnetism | 1 |
| Magnetism and Matter | 4 |
| Thermodynamics | 1 |
| Wave Optics | 2 |
| Alternating Current | 2 |
|
Semiconductor Electronics: Materials, Devices and Simple Circuits
|
4 |
| Units and Measurements | 3 |
| Mechanical Properties of Solids | 2 |
| Laws of Motion | 1 |
| Dual Nature of Radiation and Matter | 2 |
|
Electrostatic Potential and Capacitance
|
4 |
| Electromagnetic Induction | 1 |
| Ray Optics and Optical Instruments | 2 |
| Rotational Motion | 2 |
| Current Electricity | 3 |
| Atoms | 2 |
| Nuclei | 1 |
| Total | 50 |
NTA NEET Syllabus 2024 for Chemistry
Chemistry section of the NEET exam is said to be one of the most scoring section. Students must follow the standard syllabus prescribed by the NTA to master the NEET chemistry section. The chemistry accounts for 25% of the questions and marks in the NEET exam. The chemistry subject is further subdivided into three domains: Physical Chemistry, Inorganic Chemistry, and Organic Chemistry.
NEET Physical Chemistry syllabus
Unit I: Some Basic Concepts in Chemistry
Unit II: Atomic Structure
Unit III: Chemical Bonding and Molecular Structure
Unit IV: Chemical Thermodynamics
Unit V: Solutions
Unit VI: Equilibrium
Unit VII: Redox Reaction and Electro-Chemistry
Unit VIII: Chemical Kinetics
Inorganic Chemistry Syllabus for NEET
Unit IX: Classification of Elements and Periodicity in Properties
Unit X: P-Block Elements
Unit XI: d- and f- Block Elements
Unit XII: Coordination Compounds
NEET Organic Chemistry syllabus
Unit XIII: Purification and Characterization of Organic Compounds
Unit XIV: Some Basic Principles of Organic Chemistry
Unit XV: Hydrocarbons
Unit XVI: Organic Compounds Containing Halogens
Unit XVII: Organic Compounds Containing Oxygen
Unit XVIII: Organic Compounds Containing Nitrogen
Unit XIX: Biomolecules
Unit XX: Principles related to Practical Chemistry
NEET Chemistry Syllabus 2025 – Click to Download
Chemistry Chapterwise Weightage in NEET 2025
| Chemistry Chapterwise Weightage in NEET 2025 | |
Class 11 |
|
| Chapter Name | No. of Questions |
| Redox Reactions | 1 |
| Chemical Bonding and Molecular Structure | 3 |
| Some Basic Concepts of Chemistry | 3 |
| Thermodynamics | 3 |
| Organic Chemistry: Some Basic Principles and Techniques | 4 |
| Classification of Elements and Periodicity in Properties | 2 |
| Structure of Atom | 2 |
| Equilibrium | 3 |
| Hydrocarbons | 2 |
Class 12 |
|
| Electrochemistry | 2 |
|
Aldehydes, Ketones and Carboxylic Acids
|
3 |
| The d and f-Block Elements | 4 |
| Alcohols, Phenols and Ethers | 2 |
| Coordination Compounds | 3 |
| The p-Block Elements (XII) | 2 |
| Chemical Kinetics | 3 |
| Biomolecules | 1 |
| Haloalkanes and Haloarenes | 2 |
| Amines | 2 |
| Solutions | 2 |
| Principles of Qualitative Analysis | 1 |
| Total | 50 |
NEET 2025 Syllabus for Biology
This section accounts for one-half, i.e., 50% of the questions and marks. Students cannot pass the NEET UG exam without scoring high marks in the Biology section. That is why, below we have mentioned the standard syllabus (updated) for the NEET 2025 exam. The syllabus contains the topics included in the syllabus from both classes 11 and 12. Candidates must have knowledge of Biology to increase the percentage.
Class 11
- Unit I: Diversity in Living World
- UNIT II: Structural Organization in Animals and Plants
- UNIT III: Cell Structure and Function
- UNIT IV: Plant Physiology
- UNIT V: Human Physiology
Class 12
- UNIT I: Reproduction
- UNIT II: Genetics and Evolution
- UNIT III: Biology and Human Welfare
- UNIT IV: Biotechnology and Its Applications
- UNIT V: Ecology and Environment
NEET Biology Syllabus PDF – Click here to Download
NEET Biology Syllabus 2025
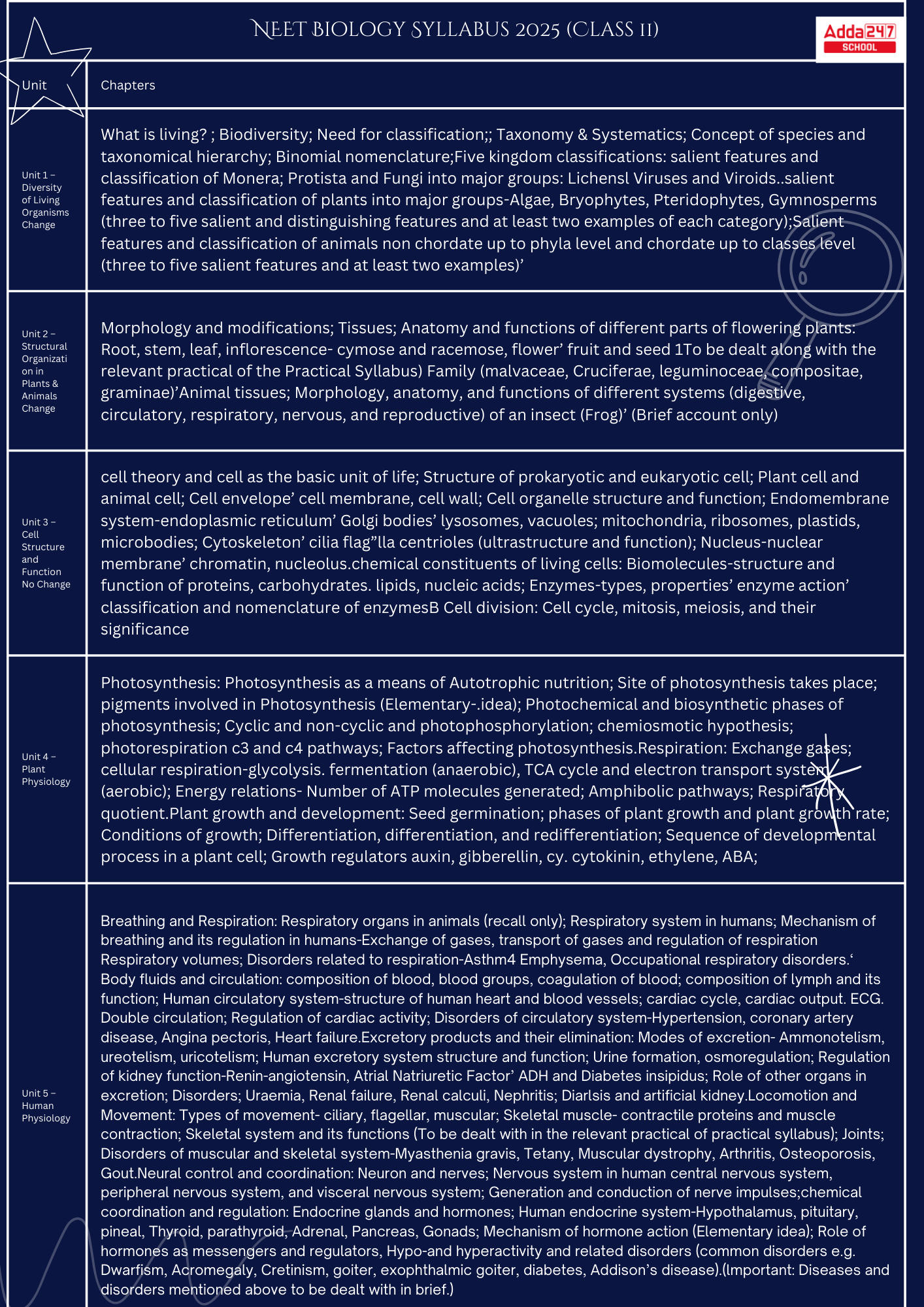
Biology Chapterwise Weightage in NEET 2025
| Biology Chapterwise Weightage in NEET 2025 | |
Class 11 |
|
| Chapter Name | No. of Questions |
| Anatomy of Flowering Plants | 3 |
| Photosynthesis in Higher Plants | 3 |
| Plant Growth and Development | 3 |
| Biological Classification | 2 |
| Cell Cycle and Cell Division | 4 |
| Cell: The Unit of Life | 4 |
| Respiration in Plants | 2 |
| Plant Kingdom | 1 |
| Structural Organisation in Animals (Animal Tissues) | 3 |
| Neural Control and Coordination | 2 |
| Chemical Coordination and Integration | 2 |
| Locomotion and Movement | 3 |
| Breathing and Exchange of Gases | 2 |
| Body Fluids and Circulation | 2 |
| Animal Kingdom | 4 |
|
Excretory Products and their Elimination
|
2 |
Class 12 |
|
|
Sexual Reproduction in Flowering Plants
|
2 |
| Biodiversity and Conservation | 5 |
| Morphology of Flowering Plants | 5 |
| Organisms and Populations | 2 |
| Principles of Inheritance and Variation | 6 |
| Microbes in Human Welfare | 1 |
| Molecular Basis of Inheritance | 6 |
| Ecosystem | 1 |
|
Biotechnology – Principles and Processes
|
5 |
| Biotechnology and its Applications | 4 |
| Human Health and Disease | 4 |
| Reproductive Health | 2 |
| Human Reproduction | 5 |
| Evolution | 4 |
| Total | 50 |
NEET 2025 Deleted Syllabus
Students must know the deleted portion from the NEET syllabus as most students will study from old textbooks that might contain the removed part of the syllabus. Knowing the deleted syllabus will lead to the preparation of irrelevant topics and waste time. The list of topics deleted from each NEET subject is given hereunder.
Syllabus Deleted from Physics
You can check revised syllabus and the topics which have been removed from the syllabus. Here is the list of topics deleted from the the NEET physics syllabus
Class 11
Unit I: Physical-world and Measurement
- Physics: Scope and excitement; nature of physical laws; Physics, technology, and society.
- Need for measurement: Length, mass, and time measurements; accuracy and precision of measuring instruments.
Unit II: Kinematics
Elementary concepts of differentiation and integration for describing motion. Scalar and vector quantities: Position and displacement vectors, general vectors, general vectors and notation, equality of vectors, multiplication of vectors by a real number; addition and subtraction of vectors. Relative velocity.
Unit III: Laws of Motion
Lubrication (under the Equilibrium of Concurrent Forces chapter)
Unit V: Rotational Motion
Momentum conservation, and centre of mass motion.
Unit VI: Gravitation
Geostationary satellites.
Unit VII: Properties of Bulk Matter/Properties of Solids and Liquids
- Shear, poisson’s ratio; elastic energy. Reynold’s number, Anomalous expansion. Specific heat capacity: Cp, Cv- calorimetry; change of state – latent heat. Qualitative ideas of Black Body Radiation, Wein’s displacement law, and Green House effect.
- Newton’s law of cooling and Stefan’s law.
Unit VIII: Thermodynamics
Heat engines and refrigerators.
Unit X: Oscillations and Waves
- Free, forced and damped oscillations (qualitative ideas only), resonance.
- Doppler effect
Class 12
Unit I: Electrostatics
Van de Graaff generator.
Unit II: Current Electricity
The flow of electric charges in a metallic conductor, Carbon resistors, colour code for carbon resistors; Potentiometer-principle and applications to measure potential difference, and for comparing emf of two cells; measurement of internal resistance of a cell.
Unit III: Magnetic Effects of Current and Magnetism
Concept of magnetic field, Oersted’s experiment. Magnetic dipole moment of a revolving electron. bar magnet as an equivalent solenoid, magnetic field lines; Earth’s magnetic field and magnetic elements. Electromagnetic and factors affecting their strengths. Permanent magnets
Unit IV: Electromagnetic Induction and Alternating Currents
LC oscillations (qualitative treatment only),
Unit VI: Optics
- Reflection and refraction of plane wave at a plane surface using wavefronts.
- Scattering of light- blue colour of the sky and reddish appearance of the sun at sunrise and sunset.
- Optical instruments: Human eye, image formation and accommodation, correction of eye defects (myopia and hypermetropia) using lenses.
- Microscopes and astronomical telescopes (reflecting and refracting) and their magnifying powers.
Unit VII: Dual Nature of Matter and Radiation
Davisson-Germer experiment (experimental details should be omitted; only conclusion should be explained).
Unit VIII: Atoms and Nuclei
Isotopes, isobars; isotones. Radioactivity- alpha, beta and gamma particles/ rays and their properties decay law.
Unit IX: Electronic Devices
Energy bands in solids (qualitative ideas only), conductors, insulators, Junction transistor, transistor action, characteristics of a transistor; transistor as an amplifier (common emitter configuration) and oscillator.
Top MBBS Colleges for NEET – Click to Check
Topics Deleted from NEET Chemistry
The following topics have been removed from the NEET chemistry syllabus.
Class 11
Unit I: Some Basic Concepts of Chemistry
General Introduction: Important and scope of chemistry.
Unit II: Structure of Atom
Atomic number, isotopes and isobars, Concept of shells and subshells, dual nature of matter and light.
Unit V: States of Matter: Gases and Liquids
- Three states of matter, intermolecular interactions, types of bonding, melting and boiling points, role of gas laws of elucidating the concept of the molecule, Boyle’s law, Charle’s law, Gay Lussac’s law, Avogadro’s law, ideal behaviour of gases, empirical derivation of gas equation. Avogadro number, ideal gas equation. Kinetic energy and molecular speeds (elementary idea), deviation from ideal behaviour, liquefaction of gases, critical temperature.
- Liquid State- Vapour pressure, viscosity and surface tension (qualitative idea only, no mathematical derivations).
Unit IX: Hydrogen
Occurrence, isotopes, preparation, properties and uses of hydrogen; hydridesionic, covalent and interstitial; physical and chemical properties of water, heavy water; hydrogen peroxidepreparation, reactions, uses and structure.
Unit X: s-Block Elements (Alkali and Alkaline Earth Metals)
Group I and group 2 elements:
- General introduction, electronic configuration, occurrence, anomalous properties of the first element of each group, diagonal relationship, trends in the variation of properties (such as ionization enthalpy, atomic and ionic radii), trends in chemical reactivity with oxygen, water, hydrogen and halogens; uses.
- Preparation and Properties of Some important Compounds:
- Sodium carbonate, sodium chloride, sodium hydroxide and sodium hydrogen-carbonate, biological importance of sodium and potassium.
- Industrial use of lime and limestone, biological importance of Mg and Ca.
Unit XI: Some p-Block Elements
Important compounds of silicon and a few uses: silicon tetrachloride, silicones, silicates and zeolites, their uses.
UNIT XIII: Organic Compounds Containing Nitrogen
Cyanides and Isocyanides- will be mentioned at relevant places.
UNIT XIV: Environmental Chemistry
Environmental pollution: Air, water and soil pollution, chemical reactions in atmosphere, smogs, major atmospheric pollutants; acid rain ozone and its reactions, effects of depletion of ozone layer, greenhouse effect and global warming-pollution due to industrial wastes; green chemistry as an alternative tool for reducing pollution, strategy for control of environmental pollution.
Class 12
Unit I: Solid State
Classification of solids based on different binding forces; molecular, ionic covalent and metallic solids, amorphous and crystalline solids (elementary idea), unit cell in two dimensional and three dimensional lattices, calculation of density of unit cell, packing in solids, packing efficiency, voids, number of atoms per unit cell in a cubic unit cell, point defects, electrical and magnetic properties, Band theory of metals, conductors, semiconductors and insulators.
Unit V: Surface Chemistry
Adsorption-physisorption and chemisorption; factors affecting adsorption of gases on solids, catalysis homogeneous and heterogeneous, activity and selectivity: enzyme catalysis; colloidal state: distinction between true solutions, colloids and suspensions; lyophillic, lyophobic multimolecular and macromolecular colloids; properties of colloids; Tyndall effect, Brownian movement, electrophoresis, coagulation; emulsions- types of emulsions.
UNIT XV: Polymers
Classification- Natural and synthetic, methods of polymerization (addition and condensation), copolymerization. Some important polymers: natural and synthetic like polyesters, bakelite; rubber, Biodegradable and non-biodegradable polymers.
UNIT XVI: Chemistry in Everyday Life
- Chemicals in medicines- analgesics, tranquilizers, antiseptics, disinfectants, antimicrobials, antifertility drugs, antibiotics, antacids, antihistamines.
- Chemicals in food- preservatives, artificial sweetening agents, elementary idea of antioxidants.
- Cleansing agents- soaps and detergents, cleansing action.
NEET Biology Deleted Syllabus 2025
The NEET Biology syllabus that has been deleted from the the standard syllabus of the NEET exam is given below.
Class 11
Unit I: Diversity in Living World
- What is Living: Three domains of life; Concept of species and taxonomical hierarchy; Tools for study of Taxonomy – Museums, Zoos, Herbaria, Botanical gardens.
- Angiosperms classification up to class, characteristic features and examples.
Unit II: Structural Organization in Animals and Plants
Animal tissues; Morphology, anatomy and functions of different systems (digestive, circulatory, respiratory, nervous and reproductive) of an insect (Frog). (Brief account only)
Unit IV: Plant Physiology
- Transport in plants: Movement of water, gases and nutrients; Cell to cell transport-Diffusion, facilitated diffusion, active transport; Plant – water relations – Imbibition, water potential, osmosis, plasmolysis; Long distance transport of water – Absorption, apoplast, symplast, transpiration pull, root pressure and guttation; Transpiration-Opening and closing of stomata; Uptake and translocation of mineral nutrients-Transport of food, phloem transport, Mass flow hypothesis; Diffusion of gases (brief mention).
- Mineral nutrition: Essential minerals, macro and micronutrients and their role; Deficiency symptoms; Mineral toxicity; Elementary idea of Hydroponics as a method to study mineral nutrition; Nitrogen metabolism-Nitrogen cycle, biological nitrogen fixation.
- Plant growth and development: Seed dormancy; Vernalization; Photoperiodism.
Unit V: Human Physiology
- Digestion and absorption; Alimentary canal and digestive glands; Role of digestive enzymes and gastrointestinal hormones; Peristalsis, digestion, absorption and assimilation of proteins, carbohydrates and fats; Caloric value of proteins, carbohydrates and fats; Egestion; Nutritional and digestive disorders – PEM, indigestion, constipation, vomiting, jaundice, diarrhea.
- Neural control and coordination: Reflex action; Sense organs; Elementary structure and function of eye and ear.
Class 12
Unit I: Reproduction
Reproduction in organisms: Reproduction, a characteristic feature of all organisms for continuation of species; Modes of reproduction – Asexual and sexual; Asexual reproduction; Modes-Binary fission, sporulation, budding, gemmule, fragmentation; vegetative propagation in plants.
Unit III: Biology and Human Welfare
Improvement in food production; Plant breeding, tissue culture, single cell protein, Biofortification; Apiculture and Animal husbandry.
Unit V: Ecology and Environment
Organisms and environment: Habitat and niche; Population and ecological adaptations
Ecosystem Patterns: Nutrient cycling (carbon and phosphorous); Ecological succession; Ecological Services fixation, pollination, oxygen release.
Environmental issues: Air pollution and its control; Water pollution and its control; Agrochemicals and their effects; Solid waste management; Radioactive waste management; Greenhouse effect and global warning; Ozone depletion; Deforestation; Any three case studies as success stories addressing environmental issues.
Click here to check List of Colleges Accepting Low NEET Score
NEET UG Syllabus 2025: New Additions
Apart from the deletion of some chapters and topics from the NEET UG syllabus, some topics have also been added to the syllabus. The list of topics added in NEET (UG) Syllabus 2025 subject-wise is given hereunder.
NEET Physics Syllabus
Unit II: Kinematics
Resolution of Vector
Unit VI: Gravitation
Motion of a satellite, time period and energy of a satellite
Unit VII: Properties of Bulk Matter/Properties of Solids and Liquids
Pressure due to a fluid column; Pascal’s law and its applications. Effect of gravity on fluid pressure.
Unit IX: Behavior of Perfect Gas and Kinetic Theory
RMS speed of gas molecules: Degrees of freedom. Avogadro’s number.
Following are the topics in the Physics syllabus of Class 12 which have been added this year.
Unit III: Magnetic Effects of Current and Magnetism
Effect of temperature on magnetic properties.
Unit VII: Dual Nature of Matter and Radiation
Dual nature of radiation.
Unit XX: Experimental Skills
Familiarity with the basic approach and observations of the experiments and activities:
1. Vernier calipers-its use to measure the internal and external diameter and depth of a vessel.
2. Screw gauge-its use to determine thickness and diameter of thin sheet/wire.
3. Simple pendulum-dissipation of energy by plotting a graph between the square of amplitude and time.
4. Metre Scale – the mass of a given object by the principle of moments.
5. Young’s modulus of elasticity of the material of a metallic wire.
6. Surface tension of water by capillary rise and effect of detergents.
7. Co-efficient of Viscosity of a given viscous liquid by measuring terminal velocity of a given spherical body.
8. Speed of sound in air at room temperature using a resonance tube.
9. Specific heat capacity of a given (i) solid and (ii) liquid by method of mixtures.
10. The resistivity of the material of a given wire using a metre bridge.
11. The resistance of a given wire using Ohm’s law.
12. Resistance and figure of merit of a galvanometer by half deflection method.
13. The focal length of;
(i) Convex mirror
(ii) Concave mirror, and
(iii) Convex lens, using the parallax method.
14. The plot of the angle of deviation vs angle of incidence for a triangular prism.
15. Refractive index of a glass slab using a travelling microscope.
16. Characteristic curves of a p-n junction diode in forward and reverse bias.
17. Characteristic curves of a Zener diode and finding reverse breakdown voltage.
18. Identification of Diode. LED,. Resistor. A capacitor from a mixed collection of such item
NEET Chemistry Syllabus
Unit II: Structure of Atom
Bohr model of a hydrogen atom – its postulates, derivation of the relations for the energy of the electron and radii of the different orbits, limitations of Bohr’s model.
Unit IV: Chemical Bonding and Molecular Structure
- Kossel – Lewis approach to chemical bond formation, the concept of ionic and covalent bonds.
- Elementary idea of metallic bonding.
- Fajan’s rule.
Unit: Chemical Thermodynamics
Fundamentals of thermodynamics: System and surroundings, extensive and intensive properties’ state functions, types of processes.
The first law of thermodynamics– Concept of work, heat internal energy and enthalpy, heat capacity, molar heat capacity; Hess’s law of constant heat summation; Enthalpies of bond dissociation, combustion’ formation, atomization. sublimation. phase transition, hydration. ionization. and solution.
The second raw of thermodynamics– Spontaneity of processes: As of the universe and AC of the system as criteria for spontaneity. Standard Gibbs energy change and equilibrium constant.
Unit VII: Redox Reactions and Electrochemistry
Electrolytic and metallic conduction, conductance and their in electrolytic solutions, molar conductivities variation with concentration: Kohlrausch’s law and its applications.
Electrochemical cells – Electrolytic and Galvanic cells, different types of electrodes, electrode potentials including standard electrode potential, half-cell and cell reactions, emf of a Galvanic Cell and its measurement: Nernst equation and its applications, Relationship between cell potential and Gibbs’ energy change: Dry cell and lead accumulator, Fuel cells.
Unit IV: Chemical Kinetics
Pressure, collision theory of bimolecular gaseous reactions (no derivation).
Unit VIII: d and f Block Elements
Transition Elements
Unit XIII: Purification and Characterization of Organic Compounds
Purification– Crystallization. sublimation, distillation, differential extraction, chromatography – principles and their applications.
Qualitative analysis– Detection of nitrogen, sulphur, phosphorus and halogens.
Quantitative analysis (basic principles only)– Estimation of carbon. hydrogen. nitrogen. halogens. sulphur. phosphorus.
Calculations of empirical formulae and molecular formulae: Numerical problems in organic quantitative analysis.
Unit XIII: Hydrocarbons
Classification of isomerism. IUPAC nomenclature, general methods of preparation, properties, and reactions.
Unit: Organic Compounds Containing Halogen
General methods of preparation, properties, and reactions; Nature of C-X bond: Mechanisms of substitution reactions.
Uses; Environmental effects of chloroform, iodoform freons, and DDT.
Unit: Principles Related to Practical Chemistry
Detection of extra elements (Nitrogen, sulphur, halogens), in organic compounds; Detection of the following functional group, hydroxyl (alcoholic and phenolic), carbonyl (aldehyde and ketones) carboxyl, and amino groups in organic compounds.
- The chemistry involved in the preparation of the following:
Inorganic compounds: Mohr’s salt. potash alum
Organic compounds: Acetanilide. p-nitro acetanilide, aniline yellow, iodoform
- The chemistry involved in the titrimetric exercises – Acids. bases and the use of indicators. oxalic-acid vs KMnO4. Mohr’s salt vs KMnO4
Chemical principles involved in the qualitative salt analysis
Cations
Anions
Chemical principles involved in the following experiments:
1. Enthalpy of solution of CuSO4
2. Enthalpy of neutralization of strong acid and strong base
3. Preparation of lyophilic and lyophobic sols
4. Kinetic study of the reaction of iodide ions with hydrogen peroxide at room temperature.
NEET Biology Syllabus
Unit II: Structural Organisation in Animals and Plants
Family (malvaceae, Cruciferae, leguminoceae, compositae, graminae)’
Unit VII: Genetics and Evolution
Molecular basis of inheritance: Protein biosynthesis
Unit VIII: Biology and Human Welfare
Health and Disease: Pathogens; parasites causing human diseases (dengue, chikungunya), Tobacco abuse
Unit X: Ecology and Environment
Biodiversity and its conservation: Sacred Groves.
How to Access and Utilize the NEET 2025 Syllabus?
To access the NEET 2025 syllabus, candidates should visit the official National Testing Agency (NTA) website where the syllabus can be downloaded in PDF format. NEET 2025 aspirants can also check the complete syllabus on this page by downloading the PDF.
After obtaining the syllabus, students should review it thoroughly to understand the exam’s scope. Aspiring candidates should incorporate the syllabus into their study plans, aligning their preparation materials and practice questions with the outlined topics.
Effectively utilizing the syllabus involves breaking down each subject into manageable sections, scheduling regular review sessions, and focusing on areas marked as changes or recent additions.
NEET 2025 Syllabus PDF by NTA Free Download
The updated syllabus for NEET UG 2025 exam will be released on the official website. So, we are providing students with the updated NEET syllabus pdf released by NMC after revision of the syllabus. If changes happen then we will update it here in the pdf. NEET 2025 Syllabus PDF Download is given below.




 NEET Revised Answer Key 2024 Out, Downlo...
NEET Revised Answer Key 2024 Out, Downlo...
 NEET Age Limit 2025, Check Category-wise...
NEET Age Limit 2025, Check Category-wise...
 NEET Exam Pattern 2025, Exam Mode, Marki...
NEET Exam Pattern 2025, Exam Mode, Marki...












