Niels Bohr Atomic Model
The niels bohr atomic model is a description of the structure of atoms, particularly hydrogen, proposed by Danish physicist Niels Bohr in 1913. The niels bohr atomic model of the atom was the first to incorporate quantum theory and was the forerunner of entirely quantum-mechanical models. It represented a significant break from earlier, classical representations. The properties of atomic electrons are described by the niels bohr atomic model and all of its successors in terms of a set of permissible (possible) values. Only when electrons abruptly leap between permissible, or stationary, states do atoms absorb or release radiation. The German-born physicists James Franck and Gustav Hertz obtained direct experimental evidence for the existence of such discrete states in 1914.
Bohr Model Class 11 and 12
Bohr model for Class 11 and 12 Students: Bohr changed his mind about the planetary electrons’ mobility to align the model with the regular patterns (spectral series) of light emitted by real hydrogen atoms. niels bohr atomic model was able to explain the series of discrete wavelengths in the hydrogen emission spectrum by restricting the orbiting electrons to a series of circular orbits with discrete radii. He argued that light only emitted from hydrogen atoms when an electron moved from an outer orbit to one closer to the nucleus. The energy lost by the electron during the sudden transition is identical to the energy of the emitted light quantum.
Niels Bohr Atomic Model Theory
Niels Bohr was a Danish physicist who made significant contributions to the understanding of atomic structure and quantum mechanics. His atomic theory, known as the Bohr model, was proposed in 1913 and played a crucial role in explaining the behavior of atoms and their spectral lines.
Key points of niels bohr atomic model theory:
- Energy Levels: Bohr’s model suggested that electrons move around the nucleus of an atom in specific energy levels or orbits. These orbits are quantized, meaning that electrons can only occupy certain discrete energy levels and not any energy level in between.
- Stationary Orbits: According to Bohr’s theory, electrons can only exist in stable, stationary orbits with fixed energy values. These orbits are sometimes referred to as “quantum shells” or “electron shells.”
- Energy Absorption and Emission: Electrons can move between energy levels by absorbing or emitting specific amounts of energy in the form of photons (particles of light). When an electron absorbs energy, it moves to a higher energy level, and when it emits energy, it falls back to a lower energy level.
- Stability of Orbits: Electrons in the innermost orbit have the lowest energy and are more stable, while those in the outer orbits have higher energy and are less stable. Electrons tend to occupy the lowest available energy levels, known as the ground state, but they can be excited to higher levels under certain conditions.
- Spectral Lines: Bohr’s model successfully explained the origin of spectral lines observed in the emission and absorption spectra of elements. When an electron moves between energy levels, it emits or absorbs photons of specific wavelengths, producing characteristic lines in the spectrum.
Though the niels bohr atomic model was groundbreaking, it had limitations and was eventually superseded by more advanced quantum mechanical models. The Bohr model worked well for simple systems like hydrogen, but it failed to explain the behavior of multi-electron atoms accurately. Modern quantum mechanics, developed in the 1920s and beyond, provided a more comprehensive understanding of the atomic structure, where electrons are described by wave functions rather than distinct orbits. Nonetheless, Bohr’s work laid the foundation for future developments in quantum theory and remains an essential milestone in the history of atomic physics.
Niels Bohr Atomic Model with Diagram
Niels bohr atomic model, proposed by Niels Bohr in 1913, was a significant advancement in understanding the structure of atoms. It was developed based on the idea of energy quantization and the observation of line spectra in the emission of light by atoms. Here’s an explanation of niels bohr atomic model along with a simplified diagram:
- Energy Levels: Bohr proposed that electrons in an atom can only occupy specific energy levels or orbits, which are represented by concentric circles around the nucleus. Each energy level has a specific energy associated with it.
- Ground State: The lowest energy level closest to the nucleus is called the ground state. In this state, the electron is in its most stable configuration and is least likely to absorb or emit energy.
- Excited States: When an electron absorbs energy, it can move to a higher energy level or an excited state. This occurs when an electron gains energy from an external source such as heat or light.
- Emission of Light: When an excited electron returns to a lower energy level, it releases the excess energy in the form of light. The emitted light has a specific wavelength and corresponds to the energy difference between the two energy levels involved.
- Fixed Orbits: According to Bohr’s model, electrons are restricted to specific orbits and cannot exist in between these energy levels. These orbits are sometimes referred to as stationary states.
- Quantized Energy: The key idea of Bohr’s model is that electrons possess quantized energy, meaning they can only have specific energy values corresponding to specific orbits. The energy levels increase as you move away from the nucleus.
- Limitations: Bohr’s model was successful in explaining certain aspects of atomic spectra but had limitations. It did not account for phenomena like electron-electron interactions and the wave-particle duality of electrons, which are better described by modern quantum mechanics.
While the actual structure of atoms is more complex than Bohr’s model suggests, it laid the foundation for the development of quantum mechanics and contributed to our understanding of atomic behavior.
Below is the Bohr atomic Model of a Nitrogen atom.
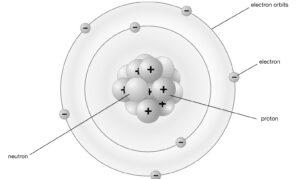
Bohr Model Formula
Bohr’s Model Formula is:
where l is the angular momentum, n is the Principle Quantum number, h is Planck’s constant.
Also, The energy and radii of electron orbits in atoms are quantized, according to Bohr, with energy for transitions between orbits given by ∆E = hf = Ei − Ef, where ∆E is the difference in energy between the beginning and final orbits and hf is the energy of an absorbed or emitted photon.
Niels bohr atomic model Postulates and Limitations
Bohr’s atomic model postulates
The niels bohr atomic model postulates are:
- Orbits, shells, and energy levels are the set circular paths electrons take around the nucleus. Stationary orbit describes the orbits.
- Each round orbit has a predetermined amount of energy, and these circular orbits are referred to as orbital shells. As long as the electrons in the fixed orbital shells continue to rotate around the nucleus, they will not emit energy.
- Integers such as n=1 or n=2 or n=3 and so on are used to represent the various energy levels. Quantum numbers are what they’re called. Quantum numbers can have a wide range of values, ranging from the lowest energy level (nucleus side n=1) to the greatest energy level.
- When electrons jump from one energy level to another, they change their energy. In an atom, electrons get the requisite energy to go from a lower to a higher energy level. When an electron loses energy, however, it shifts from a higher to a lower energy level.
- The various energy levels or orbits are represented in two ways: 1, 2, 3, 4… and K, L, M, N….. shells. The ground state of an electron is its lowest energy level.
Niels bohr atomic model Limitation
Following are the limitations of Bohr’s atomic model:
- The Heisenberg Uncertainty Principle is violated in Bohr’s atomic model. According to the Bohr atomic model theory, electrons have both a known radius and orbit, i.e., known position and momentum at the same time, which Heisenberg claims is impossible.
- When it comes to smaller atoms like hydrogen, the Bohr atomic model theory makes accurate forecasts, but when it comes to larger atoms, it makes poor spectral predictions.
- When the spectral line is divided up into fine lines in the presence of an electric field, Bohr’s atomic model fails to describe the Stark effect.
- When the spectral line is broken into numerous components in the applied magnetic field, Bohr’s atomic Model likewise fails to explain the Zeeman phenomenon.
Mention the main ideas of Bohr’s model of an atom
Niels Bohr’s atomic model, proposed in 1913, introduced several key ideas to explain the behavior of atoms and their spectra. The main ideas of Bohr’s model of an atom are as follows:
- Quantization of Energy Levels: Bohr suggested that electrons in an atom can only exist in specific, discrete energy levels or orbits. These orbits are quantized, meaning that electrons can have only certain fixed energy values and not any energy level in between.
- Stationary Orbits: Electrons move around the nucleus in stable, stationary orbits or “quantum shells.” Each orbit corresponds to a specific energy level, and electrons can transition between these orbits by absorbing or emitting energy.
- Energy Absorption and Emission: When an electron absorbs energy, it moves to a higher energy level or orbit. Conversely, when it releases energy, it falls back to a lower energy level. These energy transitions are associated with the emission or absorption of photons, packets of light energy.
- Stability of Orbits: Electrons in the innermost orbits have the lowest energy and are more stable, while those in outer orbits have higher energy and are less stable. Electrons tend to occupy the lowest available energy levels, known as the ground state, but they can be excited to higher levels under certain conditions.
- Explanation of Spectral Lines: One of the significant achievements of Bohr’s model was its ability to explain the origin of spectral lines observed in the emission and absorption spectra of elements. The discrete energy levels of the electrons in different orbits result in characteristic lines in the spectrum when electrons transition between energy levels.
- Limitations: While the Bohr model successfully explained the behavior of hydrogen and some other one-electron systems, it failed to account for the behavior of multi-electron atoms accurately. The model did not account for the wave-like behavior of electrons, which was later addressed by the development of quantum mechanics.
Bohr’s model was a pioneering step in the understanding of atomic structure and marked a significant departure from the classical physics of that time. Although it was eventually replaced by more advanced quantum mechanical models, Bohr’s contributions laid the foundation for future developments in atomic and quantum physics.
Related Post:
- Median Formula For Even, Odd, And Grouped Data
- The Largest Country In The World By Area & Population
- Value Of E In Maths And Physics
- 17 National Symbol Of India With Names List
- Father Of Mathematics In India And World
- IPPB Full Form – India Post Payments Bank
- Thank You Coronavirus Helpers: Essay, Images, Messages, Pictures
- Name The Largest Delta In The World
- Projectile Motion- Definition, Formula, Derivation, Examples








 CUET BHU Cut off 2025: Course-wise and C...
CUET BHU Cut off 2025: Course-wise and C...
 DAVV Result 2025 Out, Download Devi Ahi...
DAVV Result 2025 Out, Download Devi Ahi...
 Calicut University Timetable 2025 PDF Re...
Calicut University Timetable 2025 PDF Re...










