Lime Water
Lime water can refer to two different things: a solution made by mixing water with lime (calcium oxide or calcium hydroxide), or it can also refer to water infused with lime fruit (the citrus fruit). Here’s an explanation of both:
- Lime Water (Calcium Hydroxide or Calcium Oxide Solution):
- Lime water is a saturated solution of either calcium oxide (CaO) or calcium hydroxide (Ca(OH)2) in water. It is often used in various applications, including:
- Chemistry: Lime water is commonly used in chemistry experiments to test for the presence of carbon dioxide (CO2). When CO2 is bubbled through lime water, it reacts with the calcium hydroxide to form calcium carbonate (CaCO3), which is insoluble and gives the solution a milky appearance. This reaction is often used as a simple test for the presence of CO2 gas.
- Water Treatment: Lime water is used in water treatment processes to raise the pH of acidic water and to precipitate impurities like iron, manganese, and hardness minerals. It helps in softening water and making it less corrosive.
- Agriculture: Agricultural lime, which is essentially calcium hydroxide, is used to adjust soil pH in farming to improve crop growth.
- Construction: Lime is sometimes used in construction materials such as mortar and plaster.
- Lime Water (Infused with Lime Fruit):
- This type of lime water is simply water that has been flavored with lime juice or slices of lime fruit. It is a refreshing and citrus-flavored drink that is often served as a beverage. It can be enjoyed plain, sweetened, or even with added herbs and spices for extra flavor.
- Lime water (water infused with lime juice) is a popular choice for those looking for a healthy and hydrating drink. It provides a burst of vitamin C and a tangy, refreshing taste.
Lime Water Formula
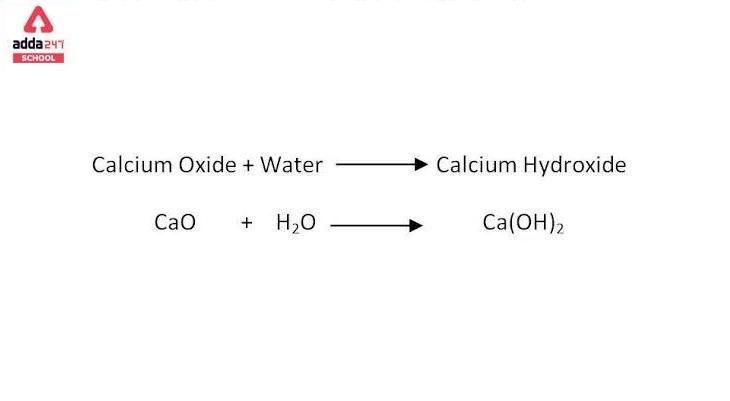
Lime water typically refers to a solution of calcium hydroxide (Ca(OH)2) in water. Calcium hydroxide is often referred to as “slaked lime” or simply “lime.” The chemical formula for calcium hydroxide is Ca(OH)2.
The formula for making lime water involves dissolving calcium hydroxide in water. Here’s the balanced chemical equation for the reaction:
Ca(OH)2 (s) + H2O (l) → Ca(OH)2 (aq)
In this equation:
- Ca(OH)2 (s) represents solid calcium hydroxide.
- H2O (l) represents liquid water.
- Ca(OH)2 (aq) represents the resulting calcium hydroxide solution in water.
To make lime water:
- Start with solid calcium hydroxide (Ca(OH)2). You can purchase this compound from chemical supply stores.
- Add the desired amount of solid calcium hydroxide to a container.
- Gradually add water to the container, stirring continuously until the calcium hydroxide is fully dissolved. The resulting solution is lime water (calcium hydroxide solution).
Be cautious when handling calcium hydroxide, as it is a strong base and can be caustic. Use appropriate safety measures, such as gloves and eye protection, when working with it.
Lime water is commonly used in various applications, including as a laboratory reagent, in water treatment processes, and for testing the presence of carbon dioxide (CO2) due to its ability to react with CO2 to form calcium carbonate (CaCO3), which causes cloudiness in the solution.
Slaked Lime
Slaked lime, often known as calcium hydroxide, is an inorganic substance having the chemical formula Ca(OH)2. When water is combined with quicklime (calcium oxide), a colourless crystal or white powder is created. It goes by a variety of names, including pickled lime, hydrated lime, caustic lime, builders’ lime, and slaked lime. Calcium hydroxide, often known as E number E526, is a chemical that is employed in a variety of processes, including the production of food. A saturated solution of calcium hydroxide is known as limewater, often known as milk of lime.
Slaked Lime Formula
The Slaked Lime Formula is Ca(OH)₂.
Slaked lime is the traditional name for calcium hydroxide. Slaked lime is a colourless crystal or white powder made up of an inorganic compound with the chemical formula Ca(OH)2. Quicklime, or calcium oxide, is combined or slaked with water to generate it. Food preparation is one of the many applications for calcium hydroxide. Limewater is sometimes known as lime milk. It’s the usual name for a calcium hydroxide saturated solution.
Read: Slope Formula
Slaked Lime Formula
Slaked lime which is Calcium Hydroxide has 1 atom of Ca and 2 hydroxide ions, which is indeed two atoms each of Oxygen and Hydrogen, and hence the Chemical Formula of Slaked lime is Ca(OH)2.
Sodium Hydroxide, Chemical Formula, Uses, Common Name
Slaked Lime Formula and Chemical Name
Water reacts with calcium oxide to produce calcium hydroxide (Ca(OH)2). When lime is mixed with water, only a little percentage of it dissolves, generating limewater, while the rest remains as a suspension known as milk of lime. Calcium hydroxide is a common industrial alkali that is also found in mortars, plasters, and cement. It’s utilised in the kraft paper process and as a sewage treatment flocculant. It can be made in the lab by combining aqueous calcium chloride and sodium hydroxide solutions. Portlandite, a mineral form, is uncommon but can be found in volcanic, plutonic, and metamorphic rocks. It’s also been reported to appear in coal-fired power plants.
Read: Work Formula
Dry Slaked Lime Formula
Calcium hydroxide has the chemical formula Ca(OH)2. It’s a type of ionic compound. Calcium loses two electrons to polyatomic hydroxide ions in this reaction. Ca+2 ions create one ionic connection with each OH- ion in slaked lime. Although the oxygen and hydrogen atoms in the polyatomic hydroxide anion have covalent connections. Between the layers of calcium hydroxides, strong hydrogen bonds exist. It has a hexagonal crystal structure as a result of this.
Slaked Lime Formula and Uses
- Lime mortar is frequently made with calcium hydroxide.
- The use of calcium hydroxide as a flocculant is one of its most common uses. It is utilised in the treatment of water and sewage. It’s also utilised in the treatment of freshwater to raise the pH so that pipes don’t corrode.
- The paper sector is another important use. Green liquor is a solution primarily of sodium carbonate and sodium sulphate created by dissolving smelt, which is the molten form of these chemicals from the recovery furnace, in the causticizing operation.
- Slaked lime is commonly utilised in the food business because of its low toxicity and mild basic characteristics.
- Calcium hydroxide is commonly chewed alongside coca leaves to preserve the alkaloid stimulants chemically accessible for body absorption.
- To preserve the alkaloid stimulants chemically available to reach the bloodstream via sublingual absorption, calcium hydroxide is frequently added to a bundle of areca nut and betel leaf called “paan.”
- Usually, calcium hydroxide is used to make lime mortar.
- The use of calcium hydroxide as a flocculant in water and sewage treatment is a prominent application.
- It produces a cleaner end product by helping to remove tiny particles from water by forming a fluffy charged solid.
- The affordability and low toxicity of calcium hydroxide make its use possible.
- Because it is self-regulating and doesn’t elevate the pH too high, it is also used in fresh water treatment to improve the pH of the water so that pipes won’t corrode when the base water is acidic.
- It has a significant use in the paper industry as an intermediate in the reaction that yields sodium hydroxide.
- The Kraft pulp-making process includes a causticizing stage that includes this conversion.
- Sodium carbonate and sodium sulphate are dissolved in green liquor, which is created by dissolving smelt, which is the molten form of these chemicals from the recovery furnace, in the causticizing process.
- Calcium hydroxide is employed as a fungicide in orchard crops.
- The application of “lime water” stops the growth of cankers brought on by the fungus Neonectria galligena.
- To avoid toxic burns caused by the extremely reactive calcium hydroxide, the trees are sprayed during the winter months while they are dormant. Regulations governing basic substances permit its use in the European Union and the United Kingdom.
- The use of calcium hydroxide in dentistry is mostly restricted to the field of endodontics.
Slaked Lime Water Formula: Properties
- Calcium hydroxide is not very water-soluble.
- An alkaline solution with a pH of around 12.5 results from the calcium hydroxide (portlandite) dissolving in clean water at room temperature.
- Chemical burns can be brought on by calcium hydroxide solutions.
- Due to a common-ion reaction with the hydroxide anion OH, its solubility dramatically reduces at high pH levels. The behaviour described here applies to cement pastes.
- Limewater is the name for aqueous solutions of calcium hydroxide, which react with acids to form medium-strength bases that can attack some metals like aluminium while shielding other metals like iron and steel from corrosion by passivating their surfaces.
The process of carbonatation, which results in the creation of insoluble calcium carbonate and causes limewater to turn milky in the presence of carbon dioxide:
| Ca(OH)2 + CO2 → CaCO3 + H2O |
Calcium hydroxide has a low water solubility and a retrograde solubility. It has a large enough dissociation in water that its solutions are basic, according to the dissolution reaction:
Ca(OH)2 → Ca2+ + 2 OH−
Calcium hydroxide dissolves in pure water at room temperature to form an alkaline solution with a pH of about 12.5. Chemical burns can be caused by calcium hydroxide solutions. Its solubility drops dramatically at high pH levels. Limewater is a medium-strength base made up of aqueous calcium hydroxide solutions. Other metals, such as iron and steel, are protected from corrosion via passivation of their surfaces. In the presence of carbon dioxide, limewater turns milky due to the creation of calcium carbonate, a process known as carbonation:
Ca(OH)2 + CO2 → CaCO3 + H2O
When calcium hydroxide is heated to 512 °C, the partial pressure of water in equilibrium with it reaches 101 kPa (normal atmospheric pressure), causing calcium hydroxide to disintegrate into calcium oxide and water:
Ca(OH)2 → CaO + H2O
It can be made in the lab by combining aqueous solutions of sodium hydroxide with calcium chloride. Although portlandite is a very uncommon mineral type, some volcanic, plutonic, and metamorphic rocks contain it. It has also been reported to appear in coal heaps that are burning. S-type stars have been shown to have the positively charged ionized species CaOH+ in their atmospheres.
Soda Lime formula
Soda lime is a chemical compound often used as a carbon dioxide absorbent in various applications, such as medical devices, laboratories, and certain industrial processes. The formula for soda lime typically consists of three main components:
- Calcium Hydroxide (Ca(OH)2): This compound provides the alkaline environment necessary for absorbing carbon dioxide (CO2). It is also known as slaked lime.
- Sodium Hydroxide (NaOH): Sodium hydroxide, or caustic soda, is another alkaline compound that helps in the absorption of CO2.
- Water (H2O): Water is essential for creating a moist environment in which the chemical reactions with CO2 can occur effectively.
The overall formula for soda lime can be represented as:
Ca(OH)2 + NaOH + H2O
The exact proportions of these components can vary depending on the specific application and manufacturer’s formulation. Soda lime is typically found in granular or pellet form and is used in devices like anesthesia machines, rebreathing systems, and in chemical processes where the removal of CO2 is necessary. It’s important to handle soda lime with care, as it can be caustic and potentially harmful if mishandled.
Quicklime Formula
The chemical formula for quicklime is CaO, which represents calcium oxide. Quicklime is a white or grayish-white solid that is produced by heating calcium carbonate (usually in the form of limestone) to a high temperature, causing it to release carbon dioxide and leave behind calcium oxide. The chemical reaction for this process is:
CaCO3 (calcium carbonate) → CaO (calcium oxide) + CO2 (carbon dioxide)
So, quicklime (CaO) is primarily composed of calcium and oxygen atoms. It is highly reactive with water and can be used for various applications, including as a construction material, in agriculture, and in chemical processes. When quicklime reacts with water, it forms calcium hydroxide [Ca(OH)2], also known as slaked lime, and releases a significant amount of heat. This reaction is exothermic and is often used in industries such as construction and water treatment.








 [Live Updates] NTA CUET Undergraduate Re...
[Live Updates] NTA CUET Undergraduate Re...
 IGNTU CUET Cutoff 2025, Check Category W...
IGNTU CUET Cutoff 2025, Check Category W...
 CGBSE Revaluation Result 2025 Announced ...
CGBSE Revaluation Result 2025 Announced ...









