Displacement Reaction
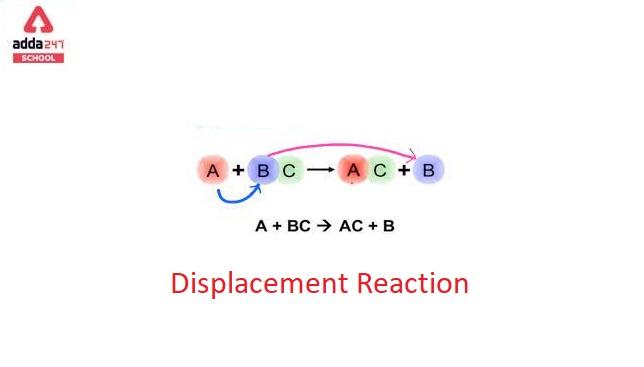
Displacement Reaction: A displacement reaction is one wherein the atom or a set of atoms is displaced by another atom in a molecule. For instance, when the iron is added to a copper sulfate solution, it displaces the copper metal. A + B-C → A-C + B. The above equation only exists when A is more reactive than B.
Displacement Reaction Definition Class 10
P+ QR=PQ + R
Displacement reaction is a very important chemical reaction, which is used in various processes like metal extraction, extraction of iron, welding, etc. In a displacement reaction, the less reactive atom in a compound is displaced with more reactive atoms.
P + QR
Here, in this compound, P, Q, and R, are different atoms, and P, is more reactive than R. hence, P replaces R in the above reaction. The most common example of displacement reaction is iron and copper sulfate.
Fe + CuSO4 FeSO4 + Cu
Here, Fe is more reactive than Cu, therefore it replaces Cu in the above reaction.
Displacement Reaction Types
There are two types of displacement reactions
- Single displacement reaction
- Double displacement reaction
- Single Displacement reaction- It is a kind of oxidation reaction, in which a single element is replaced. The element which has higher reactivity will replace the less reactive element.
- Double displacement reaction – In a double displacement reaction, both the elements of a compound react with each other and form a new element. These reactions mostly take place in an aqueous state, which facilitates precipitation and helps the elements form new bonds.
PQ + AB PA + QB
Here P, Q, A and B are four different elements that react in an aqueous solution and due double displacement reaction, the elements get exchanged and form a new element.
HCl + NaOH NaCl + H2O
Na replaces H from HCl, and H is displaced in the place of Na, with OH, forming H2O
What is Single Displacement Reaction Class 10
A single displacement reaction, also known as a single replacement reaction or a single substitution reaction, is a type of chemical reaction that occurs when one element or ion replaces another element in a compound. This reaction is characterized by the exchange of atoms or ions between two reactants, resulting in the formation of a new compound and the release of energy.
The general form of a single displacement reaction can be represented as:
A + BC → AC + B
In this equation:
- A is the element or ion that displaces B from the compound BC.
- B is the element or ion that gets displaced and is usually a metal.
- C is another element or ion bonded to B in the compound BC.
- AC is the new compound formed when A displaces B.
For example, a common single displacement reaction involves the reaction of a metal with an acid:
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
In this reaction, zinc (Zn) displaces hydrogen (H) from hydrochloric acid (HCl), resulting in the formation of zinc chloride (ZnCl2) and hydrogen gas (H2) as products.
Single displacement reactions can also occur in other types of compounds, such as when a more reactive element displaces a less reactive element in a compound. These reactions are important in understanding the reactivity of elements and predicting the products of chemical reactions.
What is Double Displacement Reaction Class 10
In chemistry, a double displacement reaction, also known as a double replacement reaction or metathesis reaction, is a type of chemical reaction in which two compounds react by exchanging ions to form two new compounds. This type of reaction typically occurs in aqueous solutions and involves the cations and anions of the compounds switching partners.
The general form of a double displacement reaction can be represented as follows:
AB + CD → AD + CB
In this reaction, A and C are usually cations (positively charged ions), while B and D are usually anions (negatively charged ions). When the reactants AB and CD come into contact, the cations A and C exchange places with each other, leading to the formation of the new compounds AD and CB.
One common example of a double displacement reaction is the reaction between sodium chloride (NaCl) and silver nitrate (AgNO3) to form sodium nitrate (NaNO3) and silver chloride (AgCl):
NaCl (aq) + AgNO3 (aq) → NaNO3 (aq) + AgCl (s)
In this reaction, the sodium ions (Na+) from sodium chloride switch places with the silver ions (Ag+) from silver nitrate, resulting in the precipitation of silver chloride (AgCl) as a solid.
Double displacement reactions are often used in analytical chemistry for the identification and separation of ions in solution, and they are commonly encountered in various chemical processes and laboratory experiments.
Example of Displacement Reaction
For every equation, it will be difficult to learn all the outcomes. To understand the displacement reactions, you should be familiar with the Reactivity series.
A displacement reaction, also known as a single replacement reaction, occurs when an element reacts with a compound and displaces another element from it. Here’s an example of a displacement reaction:
Zinc and Hydrochloric Acid:
Equation: Zn + 2HCl → ZnCl2 + H2
In this reaction, zinc (Zn) displaces hydrogen (H) from hydrochloric acid (HCl) to form zinc chloride (ZnCl2) and hydrogen gas (H2). The more reactive element, zinc, displaces the less reactive element, hydrogen, from the compound.
Keep in mind that for a displacement reaction to occur, the reacting element must be more reactive than the element it’s trying to displace from the compound.
Displacement Reaction Example for Class 10
Displacement reactions, also known as replacement reactions or single replacement reactions, are a type of chemical reaction where one element replaces another element in a compound. In these reactions, a more reactive element displaces a less reactive element from its compound. Here are some displacement reaction examples suitable for a Class 10 level of understanding:
- Zinc and Copper Sulfate: Zinc (Zn) is more reactive than copper (Cu). When zinc is added to copper sulfate (CuSO4) solution, a displacement reaction occurs:
Zn + CuSO4 -> ZnSO4 + Cu
The zinc displaces copper from copper sulfate, resulting in the formation of zinc sulfate and the deposition of copper metal.
- Magnesium and Hydrochloric Acid: Magnesium (Mg) is more reactive than hydrogen (H). When magnesium reacts with hydrochloric acid (HCl), a displacement reaction takes place:
Mg + 2HCl -> MgCl2 + H2
The magnesium displaces hydrogen from the hydrochloric acid, producing magnesium chloride and hydrogen gas.
- Iron and Copper Sulfate: Iron (Fe) is more reactive than copper (Cu). When iron is added to copper sulfate solution, a displacement reaction occurs:
Fe + CuSO4 -> FeSO4 + Cu
Iron displaces copper from copper sulfate, leading to the formation of iron sulfate and the release of copper metal.
- Aluminum and Iron(III) Oxide: Aluminum (Al) is more reactive than iron (Fe). When aluminum reacts with iron(III) oxide (Fe2O3), a displacement reaction takes place:
2Al + Fe2O3 -> Al2O3 + 2Fe
Aluminum displaces iron from iron(III) oxide, resulting in the formation of aluminum oxide (also known as alumina) and elemental iron.
- Sodium and Water: Sodium (Na) is highly reactive and can displace hydrogen from water (H2O):
2Na + 2H2O -> 2NaOH + H2
Sodium displaces hydrogen from water, producing sodium hydroxide (NaOH) and hydrogen gas.
- Potassium and Water: Similar to sodium, potassium (K) is very reactive and displaces hydrogen from water as well:
2K + 2H2O -> 2KOH + H2
Potassium displaces hydrogen from water, forming potassium hydroxide (KOH) and hydrogen gas.
Remember that these reactions demonstrate the concept of displacement, where a more reactive element takes the place of a less reactive element in a compound. Always exercise caution when performing chemical reactions, especially those involving highly reactive metals.
Read More About:








 [Live Updates] NTA CUET Undergraduate Re...
[Live Updates] NTA CUET Undergraduate Re...
 IGNTU CUET Cutoff 2025, Check Category W...
IGNTU CUET Cutoff 2025, Check Category W...
 CGBSE Revaluation Result 2025 Announced ...
CGBSE Revaluation Result 2025 Announced ...









