Aluminium Chloride
Aluminium chloride, often represented as AlCl3 is a chemical compound composed of aluminium and chlorine. Aluminium chloride is a white or pale yellow crystalline solid that is highly soluble in water and polar solvents. Aluminium chloride is widely used in various industrial and chemical applications due to its versatile properties. Here are some key points about aluminium chloride:
- Aluminium chloride Chemical Formula: AlCl3
- Aluminium chloride Molecular Weight: 133.34 g/mol
Aluminium Chloride Formula
Aluminium Chloride Formula, also known as Trichloroaluminum formula or Aluminum trichloride formula, is expressed as AlCl3, Aluminum and chlorine are combined to form aluminium chloride. Aluminium and chlorine are combined to form aluminium chloride. The chemical compound aluminium chloride formula is used to produce aluminium metal in industrial settings, but it also has a wide range of other uses, particularly as a Lewis acid. In this article, we are going to learn about Aluminium Chloride Formula, its structure, colour, Preparation and uses.
Read: Ethanol Formula: Structure, Properties and Applications
Aluminium Chloride Chemical formula
The chemical formula of aluminium chloride is AlCl3. According to the Aluminium Chloride Formula, three chloride ions are needed to create one aluminium ion. The outermost shell of aluminium has three electrons. In contrast, the outer shell of chlorine has seven electrons.
As the atomic number of Aluminium is 11, three electrons must be removed from the metal aluminium. The outer electron shell, which already has eight electrons, would then change from being the inner electron shell. In addition, the outer shell of chlorine contains seven electrons. Consequently, chlorine has a single open space.
As a result, if aluminium and chlorine were to come into contact, they would aid one another. Chlorine is the one who is ungrateful for electrons. Three electrons can be lost by a single piece of aluminium. This means that three chlorine atoms will take those three electrons.
The aluminium is ultimately stabilised by this. Additionally, the filling of the one empty space contributes to the stabilisation of the chlorine atoms.
AlCl3 Chemical Name
The chemical name for AlCl3 is aluminium chloride.
AlCl3 Compound Name
The compound AlCl3 is named as aluminium chloride.
Also Read: Modern Periodic Table of Elements with Name & Atomic mass
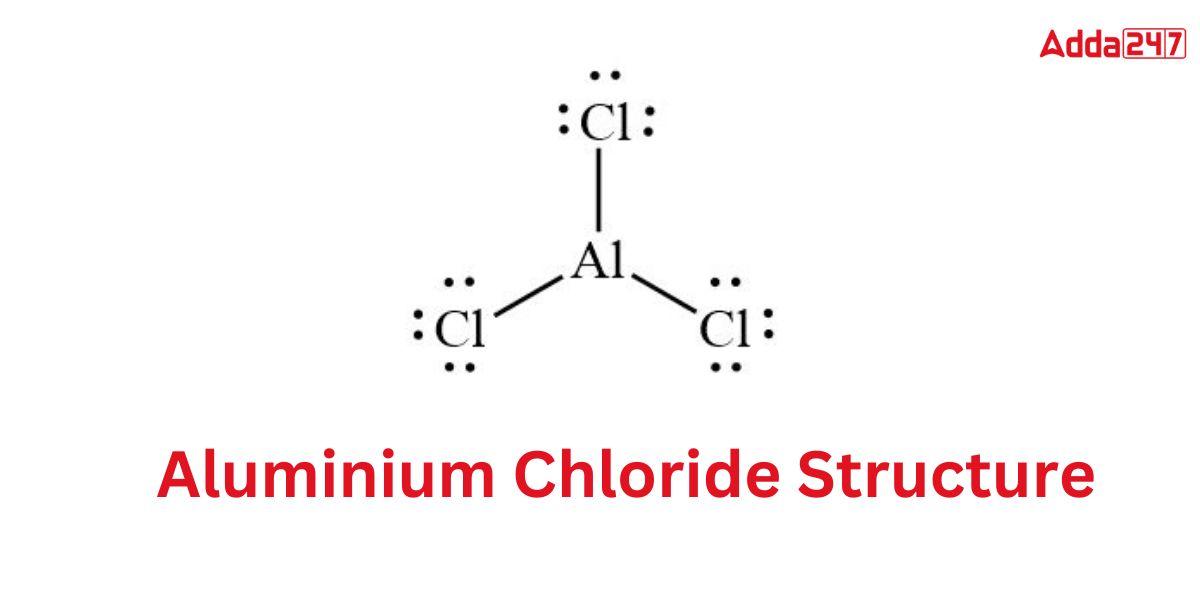
Structure of AlCl3- Aluminium Chloride Structure
The Aluminium Chloride Formula has a tendency to take on a wide variety of shapes. The condition also determines whether the Aluminium Chloride Formula is in a solid, liquid, or gaseous state. When solid, AlCl3 has a cubic, tightly packed layer structure. The Lewis structure of aluminium chloride is as follows
Aluminium Chloride Colour
Aluminum chloride is a silvery-white chemical compound. It frequently exhibits a yellow hue when contaminated with iron chloride as opposed to the white of the pure compound. It is soluble in a number of solvents, including water, chloroform, ethanol, CCl4, hydrogen chloride, and benzene, though only slightly.

Aluminium Chloride Preparation Formulas
A primary exothermic reaction between the two elements aluminium and chlorine produces aluminium chloride. There are numerous other ways to obtain aluminium chloride. Two common processes involve reacting aluminium metal with hydrogen chloride or conducting a single displacement reaction between copper chloride and aluminium metal. The preparation of AlCl3 is demonstrated by the reactions below (chemical formula of aluminium chloride).
2Al + 3Cl2 —> 2AlCl3
2Al + 6HCl → 2AlCl3 + H2
2Al + 3CuCl2 → 2AlCl3 + 3Cu
Check: Dielectric Constant Definition, Formula, Meaning in Chemistry
Aluminium Chloride Formula Unit Mass
Aluminium Chloride Properties
The properties of Aluminium chloride are listed below
- At different temperatures, Aluminium chloride can be solid, liquid, or gaseous.
- In a molten state, aluminium chloride is a poor conductor of electricity.
- Aluminum chloride is actually white in colour, but when iron trichloride contaminants are present, it takes on a yellowish colour.
- Both it’s melting and boiling points are extremely low.
- Aluminum chloride is not explosive or flammable.
- Aluminum chloride reacts strongly with both water and base.
- It can only turn into liquid at pressures and temperatures greater than 2.5 atm and 190 °C, respectively.
- It is an industrial catalyst and a Lewis acid.
- Aluminum chloride disintegrates in water, benzene, chloroform, and carbon tetrachloride.
Aluminium Chloride Uses
- The most common use of aluminium chloride (AlCl3) is as a catalyst for various chemical reactions.
- It frequently participates in the Friedel-Crafts process, which also involves acylations and alkylations. In the synthesis of anthraquinone from phosgene and benzene, it is utilised.
- Aluminum chloride can be used to introduce or attach aldehyde groups to aromatic series or rings. Consider the Gatterman-Koch reaction, which involves the removal of a chloride ion from a solution using a Lewis acid (aluminium chloride).
- Additionally, it is used in isomerization and light molecular-weight hydrocarbon polymerization processes. Examples include producing ethylbenzene and dodecylbenzene for use in detergents.
- Aluminium chloride is a common ingredient in the production of paints, lubricants, wood preservatives, and rubber.
- Aluminium chloride is widely used in pesticides and pharmaceuticals.
Aluminium Chloride ka Formula
Aluminium Chloride Formula in Hindi:एल्युमिनियम क्लोराइड फॉर्मूला, जिसे ट्राइक्लोरोएल्युमिनियम फॉर्मूला या एल्युमिनियम ट्राइक्लोराइड फॉर्मूला के रूप में भी जाना जाता है, को AlCl3 के रूप में व्यक्त किया जाता है, एल्युमिनियम और क्लोरीन को मिलाकर एल्युमिनियम क्लोराइड बनाया जाता है। एल्युमीनियम और क्लोरीन को मिलाकर एल्युमिनियम क्लोराइड बनाया जाता है। रासायनिक यौगिक एल्यूमीनियम क्लोराइड सूत्र का उपयोग औद्योगिक सेटिंग्स में एल्यूमीनियम धातु का उत्पादन करने के लिए किया जाता है, लेकिन इसके अन्य उपयोगों की एक विस्तृत श्रृंखला भी है, विशेष रूप से लुईस एसिड के रूप में।
Aluminium Chloride ka Sutra
एल्युमिनियम क्लोराइड का रासायनिक सूत्र AlCl3 है। एल्युमिनियम क्लोराइड फॉर्मूला के अनुसार, एक एल्युमिनियम आयन बनाने के लिए तीन क्लोराइड आयनों की आवश्यकता होती है। एल्युमीनियम के सबसे बाहरी खोल में तीन इलेक्ट्रॉन होते हैं। इसके विपरीत, क्लोरीन के बाहरी आवरण में सात इलेक्ट्रॉन होते हैं।
चूंकि एल्युमीनियम की परमाणु संख्या 11 है, धातु एल्यूमीनियम से तीन इलेक्ट्रॉनों को हटाया जाना चाहिए। बाहरी इलेक्ट्रॉन खोल, जिसमें पहले से ही आठ इलेक्ट्रॉन हैं, तब आंतरिक इलेक्ट्रॉन खोल होने से बदल जाएगा। इसके अलावा, क्लोरीन के बाहरी आवरण में सात इलेक्ट्रॉन होते हैं। नतीजतन, क्लोरीन के पास एक ही खुली जगह है।
नतीजतन, अगर एल्यूमीनियम और क्लोरीन संपर्क में आते हैं, तो वे एक दूसरे की सहायता करेंगे। क्लोरीन वह है जो इलेक्ट्रॉनों के लिए कृतघ्न है। एल्युमीनियम के एक टुकड़े से तीन इलेक्ट्रॉन खो सकते हैं। इसका मतलब है कि तीन क्लोरीन परमाणु उन तीन इलेक्ट्रॉनों को ले लेंगे।
इसके द्वारा एल्यूमीनियम अंततः स्थिर हो जाता है। इसके अतिरिक्त, एक खाली स्थान को भरने से क्लोरीन परमाणुओं के स्थिरीकरण में योगदान होता है।









 CUET BHU Cut off 2025: Course-wise and C...
CUET BHU Cut off 2025: Course-wise and C...
 DAVV Result 2025 Out, Download Devi Ahi...
DAVV Result 2025 Out, Download Devi Ahi...
 Calicut University Timetable 2025 PDF Re...
Calicut University Timetable 2025 PDF Re...










