Ethanol, also known as ethyl alcohol or grain alcohol, is a colorless, volatile, and flammable liquid with a chemical formula of C2H5OH. It is a common ingredient in alcoholic beverages and is widely used as a solvent, fuel, and antiseptic.
Ethanol Formula in Chemistry
Ethanol formula in chemistry: In this article, we are going to learn about ethanol formula with its structure and chemical properties. Ethanol, also referred to as ethyl alcohol, is a basic form of alcohol.EtOH is another name for ethanol or ethyl alcohol. Most alcoholic beverages typically contain ethanol. In 1808 Nicolas-Théodore de Saussure discovered the chemical formula for ethanol, and in 1858 Archibald Scott Couper provided a structural formula for ethanol. The ethanol formula and its structure are described here with explanations.
Read: Modern Periodic Table of Elements with Name & Atomic mass
Formula of Ethanol Name
The formula of ethanol C2H5OH and The chemical name of Ethanol is Ethyl Alcohol. In organic chemistry, the methyl and methylene groups combine to generate the ethyl group, abbreviated Et. For this reason, the structure of ethanol can be written as EtOH. Alcohol is a colorless liquid that is soluble in water and has a faint aroma. When left in an open container, it quickly evaporates since it is volatile and flammable. Ethanol is a chemical compound that is also referred to as Absolute alcohol, alcohol, cologne spirit, drinking alcohol, methyl carbinol, ethylic alcohol, ethyl hydrate, ethyl hydroxide, and ethane monoxide.
History of Ethanol
The use of ethanol as a fuel dates back to ancient times, when it was used as a source of heat and light. It wasn’t until the late 19th century that ethanol began to be used as a fuel for engines. In the early 20th century, ethanol was widely used as a fuel for automobiles, but the widespread availability of cheap petroleum-based fuels led to a decline in ethanol use. In recent years, however, there has been a renewed interest in ethanol as a renewable fuel source.
Ethanol Formula
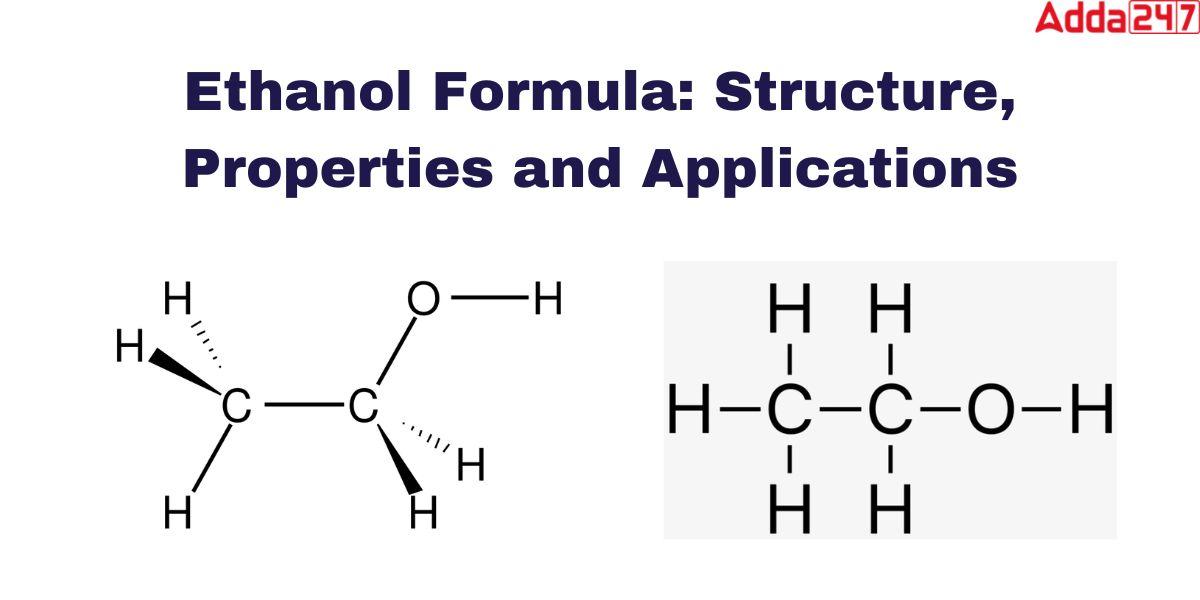
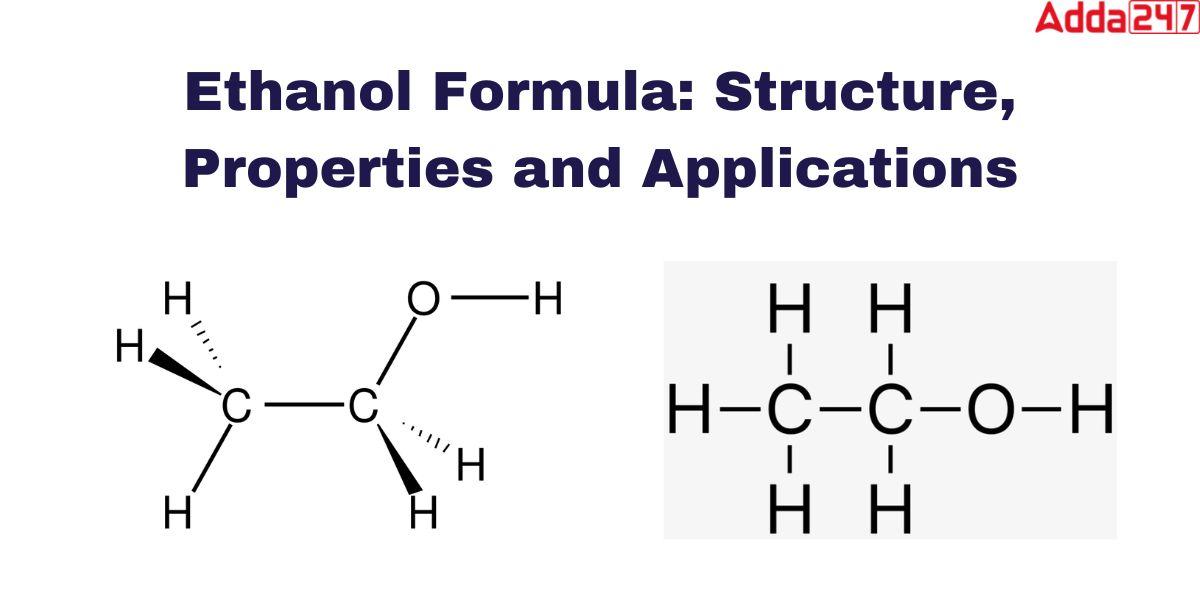
The symbol we use to denote Ethanol is C2H5OH. As a hydrocarbon component of organic chemistry Ethanol formula contains carbon, hydrogen, and oxygen. The formula of Ethanol or Ethyl alcohol is very simple. The chemical formula for ethanol is CH3CH2OH, It is easy to understand how molecules come into being. The oxygen of the hydroxyl group (OH) links with the methyl group’s (CH3) and methylene group’s (CH2) carbons.
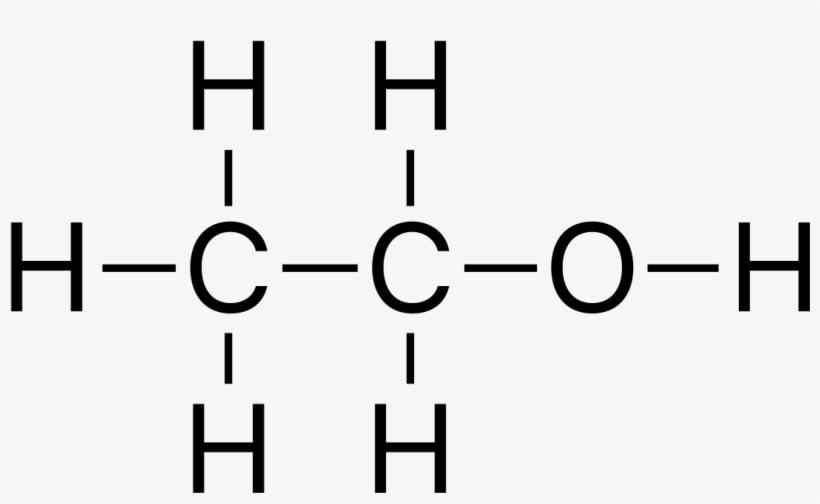
Ethanol Structure
Ethanol, commonly known as ethyl alcohol, refers to a class of organic compounds known as alcohols. The chemical formula for ethanol is C2H5OH or CH3CH2OH, and it is a mixture of the elements carbon, hydrogen, and oxygen. A molecule of ethanol contains carbon atoms that are sp3 hybridized, or free to rotate. is shown as-
Ethyl Alcohol Formula Molecular Mass
The chemical ethyl alcohol formula is C2H5OH or CH3CH2OH
1 mol of ethanol comprises 1 mol of oxygen atoms (1 mol = 15.9994g), 6 mol of hydrogen atoms (6 mol = 1.0079g), and 2 mol of carbon atoms (2 mol = 12.011g = 24.022g) in 1 mol.
Hence Ethanol has a molecular mass of 46.069 g/mol or 46.07 g/mol.
Properties of Ethanol
Ethanol, also known as ethyl alcohol, is a clear, colorless liquid that is commonly used as a solvent, fuel, and intoxicating agent. It is a simple alcohol with the chemical formula C2H5OH, and is produced by the fermentation of sugars or by synthetic processes. Ethanol is a highly versatile substance with a wide range of physical and chemical properties, which make it useful in many different applications.
Physical Properties of Ethanol
1. Solubility
One of the most important properties of ethanol is its solubility. Ethanol is highly soluble in water, which makes it an effective solvent for many substances, including polar and non-polar compounds. This property makes it useful in the production of many different types of products, from pharmaceuticals and cosmetics to gasoline and cleaning agents. Ethanol is also soluble in many organic solvents, such as acetone and ether, which expands its usefulness in different applications.
2. Volatility
Another important property of ethanol is its volatility. Ethanol has a relatively low boiling point of 78.4°C, which means it evaporates easily at room temperature. This property makes it useful as a fuel, as it can be easily vaporized and burned to produce energy. It also makes it useful in the production of many consumer products, such as perfumes, colognes, and air fresheners, where its volatile nature allows it to easily disperse its fragrance.
3. Disinfectant
Ethanol is also a good disinfectant, due to its ability to denature proteins and dissolve lipids. This property makes it useful in the production of many cleaning agents and sanitizers, including hand sanitizers and surface disinfectants. Ethanol is also used as a preservative in many products, as it helps prevent the growth of bacteria and other microorganisms.
4. Flammable
Ethanol is also flammable, which makes it a potential fire hazard. It is important to handle ethanol carefully and avoid exposure to open flames or sparks. Ethanol vapors can ignite easily, and the liquid can also burn if it comes into contact with a flame.
5. Effect on Human Body
In addition, ethanol has a unique effect on the human body, as it is a central nervous system depressant. When consumed in small amounts, ethanol can produce feelings of relaxation and euphoria. However, when consumed in excess, it can cause a range of negative effects, including impaired judgment, slurred speech, and loss of coordination. Long-term use of ethanol can also lead to liver damage and other health problems.
Ethanol is a highly versatile substance with a wide range of properties that make it useful in many different applications. Its solubility, volatility, disinfectant properties, and flammability make it a valuable ingredient in many consumer products, while its effects on the human body make it a popular recreational drug. However, it is important to handle ethanol with care and to use it responsibly, to avoid the potential hazards associated with its use.
Chemical Properties of Ethanol
In addition to its physical properties, ethanol also has a range of chemical properties that make it a useful substance in many applications. Here are some of the most important chemical properties of ethanol:
1. Reactivity with acids:
Ethanol is a weak acid and can react with stronger acids to form esters. This reaction is commonly used in the production of perfumes and other fragrances.
2. Reactivity with bases:
Ethanol is also reactive with strong bases and can be used to make soaps, detergents, and other cleaning agents.
3. Flammability:
Ethanol is highly flammable and can easily catch fire when exposed to a flame or spark. This makes it an effective fuel for engines and other combustion engines.
5. Oxidation:
Ethanol is easily oxidized, meaning that it can be converted into other chemical compounds through the addition of oxygen. This property makes it useful in the production of other chemicals, including acetic acid and ethylene.
6. Solvent properties:
Ethanol is an excellent solvent for a wide range of organic compounds, including oils, fats, and resins. This makes it useful in the production of paints, varnishes, and other coatings.
7. Toxicity:
While ethanol is generally safe for human consumption in small quantities, it can be toxic in larger doses. This is because ethanol is metabolized by the liver into acetaldehyde, which is toxic to the body.
8. Sterilization:
Ethanol has strong antiseptic properties and can be used to sterilize equipment and surfaces in hospitals, laboratories, and other settings.
Overall, the chemical properties of ethanol make it a versatile and useful substance in many different industries. Its reactivity with acids and bases, flammability, oxidation properties, solvent properties, toxicity, and sterilization abilities make it an essential chemical compound in many manufacturing processes.
Uses of Ethanol
Ethanol has a wide range of uses and applications.
- The most common use of ethanol is as a fuel for automobiles. Ethanol can be used as a fuel by itself, or it can be blended with gasoline to increase octane and reduce emissions.
- Ethanol is also used as a solvent in a variety of applications, including pharmaceuticals, personal care products, and cleaning products.
- In addition, ethanol is used as a disinfectant in hospitals and other medical settings.

Ethanol Alcohol formula: Applications
One of the most prevalent organic substances found in both industrial and consumer goods is ethanol.
- Despite being a very straightforward chemical, ethanol has a number of applications. Households, antiseptic cleaning solutions, and even varnishes use it.
- Vinegar is made from ethanol and is used in households for a variety of purposes since it is a weak solution of ethanoic acid.
- Ethanol serves as a fuel, a superior solvent, and a crucial raw element for the production of other chemicals. In addition to being added to automotive gasoline, it is employed as a solvent and in the synthesis of other organic compounds.
- Drugs, plastics, lacquers, polishes, plasticizers, and cosmetics are all made with ethanol. Ethanol is used in medicine as an antidote for ethylene glycol or methanol overdose as well as a topical antiinfective.
- This aliphatic alcohol is mostly used in industry as a solvent and as an intermediary in the synthesis of other compounds.
- Alcoholic Beverages, colognes, mouthwashes, liniments, perfumes, aftershaves, and various rubbing alcohols are examples of commercial products that include ethanol.
Production of Ethanol
Ethanol is typically produced through the fermentation of sugars, which can be derived from a variety of sources, including grains, fruits, and vegetables. The most common sources of ethanol are corn, wheat, and sugarcane. The process of producing ethanol involves several steps, including milling the grain, converting the starch to sugar, fermenting the sugar to alcohol, and distilling the alcohol to remove impurities and increase its concentration.

Ethanol production has been steadily increasing over the years, driven by the need for a cleaner alternative to fossil fuels and to reduce greenhouse gas emissions. In this article, we will discuss the production of ethanol, including its benefits, production methods, and applications.
Benefits of Ethanol Production
There are several benefits associated with the production of ethanol. Some of the key benefits include:
- Renewable: Ethanol is derived from renewable sources such as corn, sugarcane, and other biomass feedstocks. This makes it a sustainable fuel option that can help reduce our reliance on fossil fuels.
- Clean-burning: Ethanol burns cleaner than gasoline, which means it produces fewer emissions that contribute to air pollution and climate change.
- Economic: Ethanol production creates jobs and helps support rural economies by providing a market for agricultural products.
- Energy Security: Ethanol production reduces our dependence on foreign oil, which can help improve our energy security.
Production Methods of Ethanol
There are two primary methods for producing ethanol: fermentation and distillation.
Production Methods of Ethanol: Fermentation
This method involves converting sugar into ethanol through the use of yeast. The sugar can be derived from a variety of sources, including corn, sugarcane, and other biomass feedstocks. The process begins by mashing the feedstock and then adding yeast to the mash. The yeast then converts the sugar into ethanol through a process called fermentation. Once the fermentation process is complete, the ethanol is separated from the remaining materials and purified.
Production Methods of Ethanol: Distillation
This method involves separating the ethanol from water through the use of heat. The process begins by adding the ethanol-water mixture to a still, which is heated to a temperature of around 173 degrees Fahrenheit. The heat causes the ethanol to vaporize and separate from the water. The ethanol vapor is then condensed back into a liquid form and purified.
Ethanol ka Formula
इथेनॉल का रासायनिक नाम एथिल अल्कोहल है। कार्बनिक रसायन विज्ञान में, मिथाइल और मिथाइलीन समूह एथिल समूह, संक्षिप्त एट उत्पन्न करने के लिए गठबंधन करते हैं। इस कारण से एथेनॉल की संरचना को EtOH के रूप में लिखा जा सकता है। अल्कोहल एक रंगहीन तरल है जो पानी में घुलनशील होता है और इसमें हल्की सुगंध होती है। जब एक खुले कंटेनर में छोड़ दिया जाता है, तो यह वाष्पशील और ज्वलनशील होने के कारण जल्दी से वाष्पित हो जाता है। इथेनॉल एक रासायनिक यौगिक है जिसे एब्सोल्यूट अल्कोहल, अल्कोहल, कोलोन स्पिरिट, ड्रिंकिंग अल्कोहल, मिथाइल कार्बिनोल, एथिल अल्कोहल, एथिल हाइड्रेट, एथिल हाइड्रॉक्साइड और ईथेन मोनोऑक्साइड भी कहा जाता है।
Ethyl Alcohal Formula in Hindi
इथेनॉल को निरूपित करने के लिए हम जिस प्रतीक का उपयोग करते हैं वह C2H5OH है। कार्बनिक रसायन विज्ञान के हाइड्रोकार्बन घटक के रूप में इथेनॉल सूत्र में कार्बन, हाइड्रोजन और ऑक्सीजन शामिल हैं। इथेनॉल या एथिल अल्कोहल का सूत्र बहुत सरल है। इथेनॉल का रासायनिक सूत्र CH3CH2OH है, यह समझना आसान है कि अणु कैसे अस्तित्व में आते हैं। हाइड्रॉक्सिल समूह (OH) का ऑक्सीजन मिथाइल समूह (CH3) और मिथाइलीन समूह (CH2) कार्बन से जुड़ता है।








 CUET UG Final Answer Key 2025 Revised, D...
CUET UG Final Answer Key 2025 Revised, D...
 DU Cut off 2025, Delhi University Expect...
DU Cut off 2025, Delhi University Expect...
 OUAT Result 2025 OUT @ouat.nic.in: Check...
OUAT Result 2025 OUT @ouat.nic.in: Check...









