Acetylene Formula: Ethyne is another name for acetylene. Acetylene is the most basic alkyne and a hydrocarbon. The acetylene formula is C2H2. It is an unsaturated chemical. Two bonds join the two carbon atoms that comprise it. Despite the fact that it is unstable in its most basic form, it is used as a solution. Acetylene is widely employed in illuminant, oxyacetylene welding, cutting, and soldering of metals; signaling; precipitating copper, in particular, and acetaldehyde; and acetic acid manufacturing. In this post, we will go through the Acetylene Formula, Structure, Hybridization, Properties, Applications, and more.
Acetylene Formula
What is Acetylene? Acetylene, also known as Narcylen and Vinylene, has the chemical formula C2H2. Vinylene is a colorless gas with a faint ether-like odor. It dissolves easily in chloroform, water, acetone, and benzene. It is slightly soluble in carbon disulfide and alcohol. It is lighter than air and has the ability to ignite. Long-term exposure to flame or heat may cause the containers to break. When exposed to pressures of more than 15 pounds per square inch, pure acetylene explodes rapidly, whether in its liquid or solid state.
Acetylene Formula Structure
Acetylene is the most basic alkyne molecule, which is a functional group defined by scientists as possessing triple bonds.
- Acetylene’s chemical formula is C2H2, and its prolonged formula is CH≡CH.
- It is composed of two carbon atoms connected by double bonds.
- In acetylene, two hydrogen atoms are connected to each of the two triple-bonded carbon atoms.
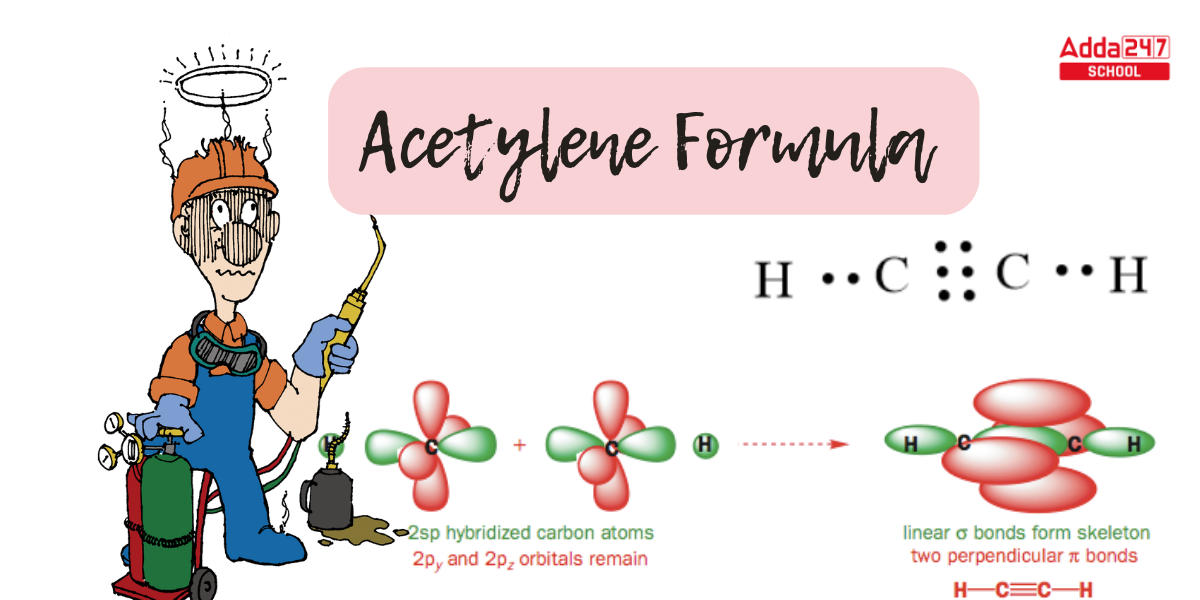
- Its molecules are 180 degrees linear, with its carbon atoms hybridized sp as a result. Furthermore, both carbons contain two sp orbitals, one of which links to the hydrogen and the other to the carbon’s simple bond.
- On the other hand, the triple bond, which consists of two bonds that form without hybridization between the four P orbitals, is orthogonal to the linear system.

Acetylene Formula Ethene
Ethene’s molecular formula will be C2H4. Ethene is an unsaturated hydrocarbon with two carbon atoms and four hydrogen atoms linked by a double bond.
- The hydrocarbons with the general formula CnH2n, where n is the number of carbon atoms, are known as alkenes.
- The electron dot structure is created by remembering the Octet rule, which states that throughout the synthesis of a molecule, an atom of a specific element can gain, lose, or share electrons until it reaches the stable electronic configuration of 8 electrons in its valence shell.
- Carbon contains four valence electrons, whereas hydrogen has just one.
- Ethene’s electron dot structure is seen below.
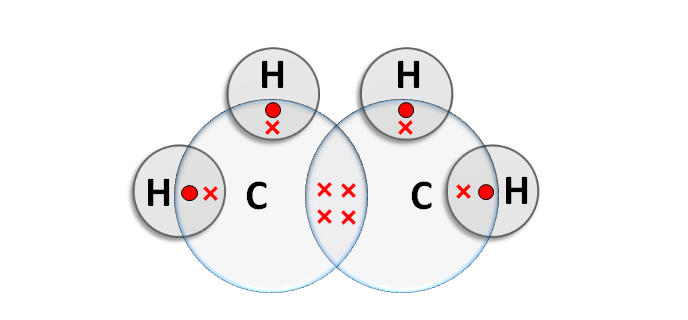
Acetylene Formula Benzene
It is an aromatic ring structure in which carbons are linked together by alternate double bonds and hydrogen is linked to carbons.C6H6 is the chemical formula for benzene.
- Benzene has an average atomic mass of 78.112 g. Benzene contains hydrogen atoms.
- Since the double bonds within this structure are mostly separated by a single bond, it is considered to contain conjugated double bonds.
- A circle is used as an auxiliary sign inside the hexagon that indicates six pi electrons.
- The six electrons in carbon are divided into two subshells, K and L. In this case, the K subshell is already full, but the L subshell only has four electrons, implying that the L subshell needs four more electrons to complete its octet.
- Because a hydrogen atom has only one electron, which is positioned in the K subshell and can only have two electrons, hydrogen only needs one extra electron to make up its octet. As a result, each carbon atom forms a single bond with one hydrogen atom.
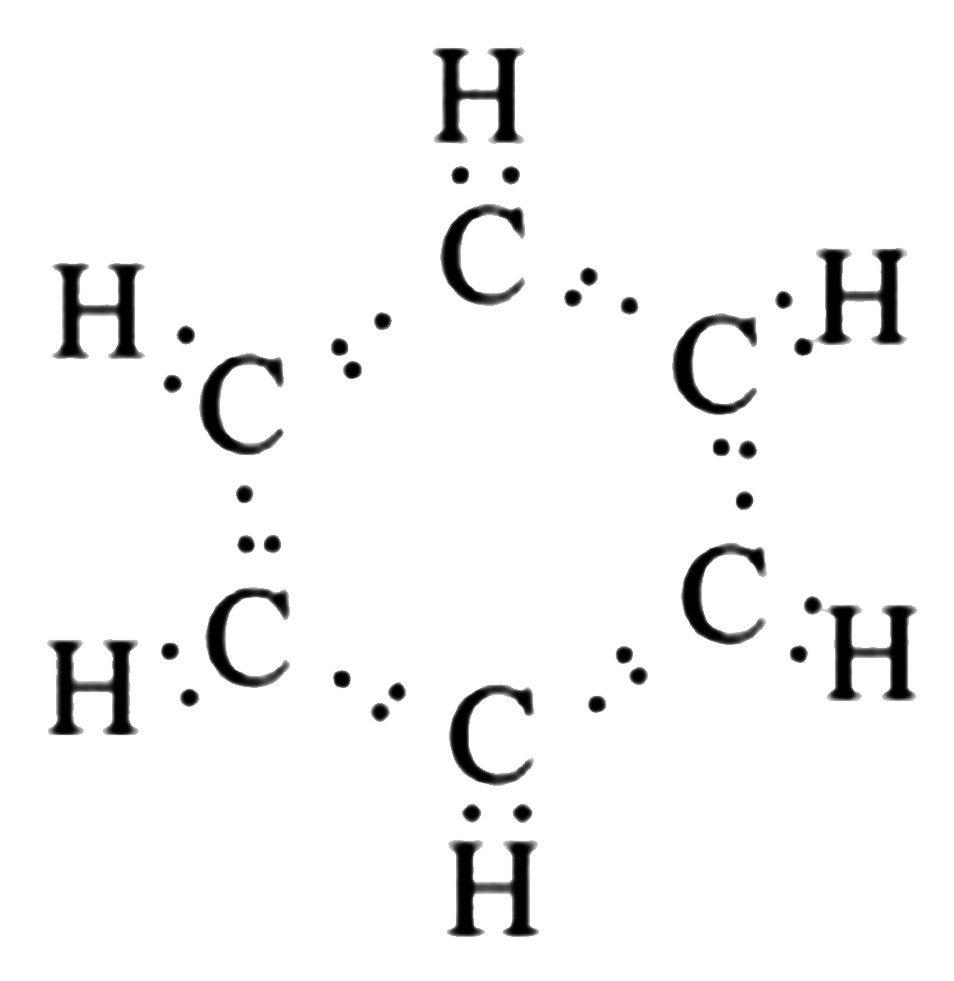
Acetylene Formula Hybridization
Triple-bonded groups such as alkaline compounds and nitriles benefit from the hybrid orbital notion. Here we will observe the structure of acetylene, the most basic alkyne.
- This molecule is linear in the sense that all four atoms are in a straight line. The carbon-carbon triple bond has a length of just 1.20. Both carbons are sp-hybridized in the hybrid orbital image of acetylene.
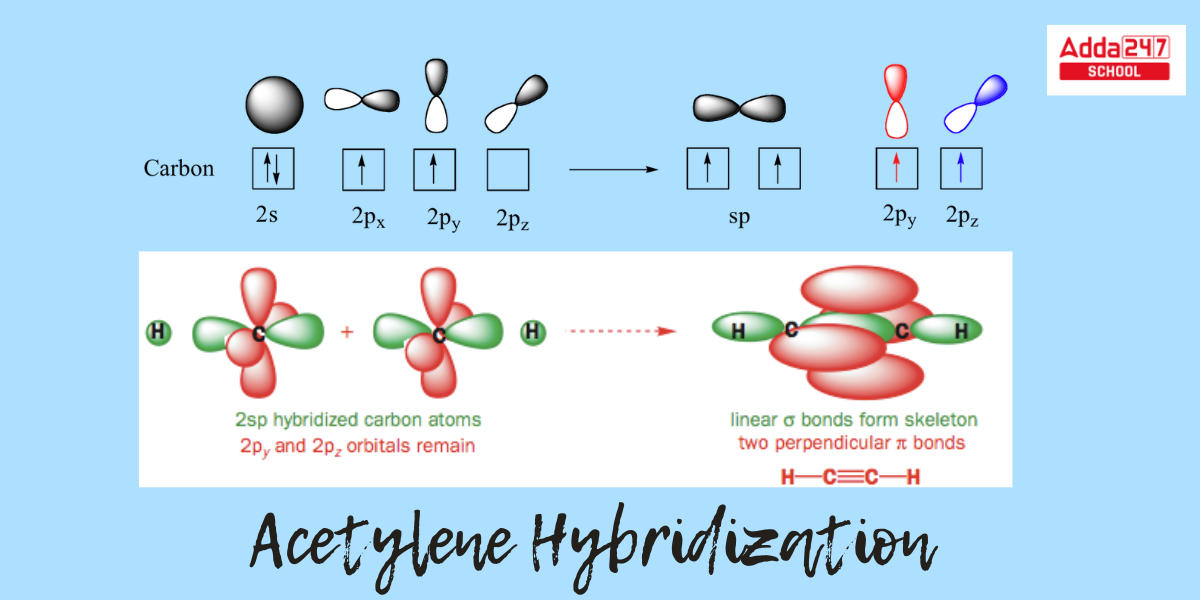
- The 2s orbital joins with the 2px orbital in a sp-hybridized carbon to generate two sp hybrid orbitals that are aligned at an angle of 180° with respect to each other (e.g. along the x-axis).
- The 2py and 2pz orbitals remain unhybridized and are perpendicular to the y and z axes, respectively.
- Carbon possesses one unpaired electron in the 2 s orbital and one lone pair in the 2 p orbital. There are two unpaired electrons in the s p orbital after hybridization, a single unpaired electron in the 2 py orbital, and one unpaired electron in the 2 p z orbital.
- The s p orbitals are parallel to the 2 p y orbitals. The 2 P z orbital is perpendicular to the page’s plane.
- The C-C sigma bond is created by the overlap of one sp orbital from each carbon with a 1s orbital from hydrogen, whereas the two C-H sigma bonds are generated by the overlap of the second sp orbital from each carbon with a 1s orbital from hydrogen.
- Each carbon atom continues to possess two half-filled 2py and 2pz orbitals that are perpendicular to each other and to the sigma bond line. Between the carbons, these two perpendicular pairs of p orbitals produce two pi bonds, resulting in a triple bond (one sigma bond + two pi bonds).
Acetylene Formula Lewis Structure
The core of the acetylene Lewis structure contains both carbon atoms because they have a larger valency (total: 8) than the hydrogen atoms (total: 2). As a result, both carbon atoms occupy the central location, with hydrogen atoms distributed surrounding them.
- The outer shell of the carbon atom contains four valence electrons. There are two carbon atoms in ethyne. As a result, each Carbon atom in C2H2 has eight valence electrons.
- The outer shell of a hydrogen atom contains one valence electron. As a result, Hydrogen atoms have two valence electrons.
- Valence electrons in C2H2 = 8 + 2 = 10, resulting in a total of ten valence electrons in the molecule.
- All of the atoms’ octets have been fully formed in Lewis structure, and there are no single pairs of electrons in the molecule.
- Both hydrogen atoms will share one of the carbon atom’s valence electrons and form a bond. The duplets of both hydrogen atoms are now finished.
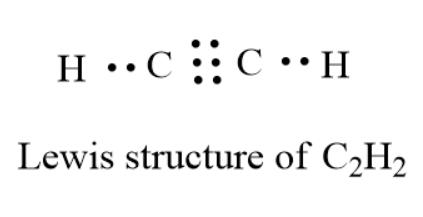
- However, the octet of carbon atoms is incomplete. To achieve a stable structure, the Carbon atoms will create a triple bond to share their remaining three valence electrons.
- A total of six valence electrons are utilized to establish a triple bond between both carbon atoms, and four electrons are used to build a single connection between carbon and hydrogen atoms.
Acetylene Properties
Let’s look at some of the most important physical properties and chemical properties of acetylene.
Acetylene Physical Properties
- Acetylene, in its purest form, is an odorless, colorless gas with a garlic-like odor.
- To ship, we dissolve any compound with acetone.
- Acetylene has a molar mass of 26.038 g/mol.
- Acetylene has melting and boiling temperatures of 80.8 °C and -84.7 °C, respectively.
- Acetylene containers can explode violently if exposed to heat or fire for an extended period of time.
- Acetylene dissolves in acetone, water, benzene, and chloroform.
- Acetylene has a density of 1.097 kg/m3 and can be measured.
- Carbon disulfide and ethanol are both weakly soluble in it.
Acetylene Chemical Properties
Due to the electrons in the C-C triple bond, acetylene is a highly reactive chemical molecule. That is why acetylene is such an excellent nucleophile. The following are the chemical properties of acetylene.
- It can undergo a wide range of reactions to yield commercial goods such as acrylic acid, acetylide, alcohol, and a vinyl compound.
- When combined with a metal like copper, it can also be used to produce organometallic compounds.
- Acetylene and sodium interact in a reaction to generate Sodium hydroxide and hydrogen.
Na + C2H2 → NaHC2 +H2
- When acetylene is treated with HBr, the result is ethylidene bromide.
CH≡CH+ HBr → CH2 = CHBr → CH3 CHBr2 (Ethylidene Bromide)
- CH3CHCI2 is generated when acetylene interacts with HCI.
C2H2 + HCI → CH2 = CHCI
CH2 = CHCI + HCI → CH3CHCI2
Where Acetylene can be found in Nature?
A few bacteria use Acetylene as the primary source of acetaldehyde synthesis. This substance can also be found in natural gas and oil wells, along with crude oil and other gases. Furthermore, it is a component of the solar planet’s atmosphere.
Acetylene Preparation
Water reacts with calcium carbide in the laboratory to produce acetylene. It was made possible by a reaction identified in 1862 by Friedrich Wohlerthe.
Method of Acetylene Preparation:
- In a conical flask, place a few bits of calcium carbide. Using the dropping funnel, add a few drops of water.
- Acetylene gas is created and collected over water via water displacement downward. The hydrolysis of the calcium carbide reaction is as follows:
CaC2+2H2O→Ca(OH)2+C2H2
- The reaction depicted above occurs at an extraordinarily high temperature, around 2000 0C, using an electric arc furnace.
- Impurities such as phosphine, hydrogen sulfide, ammonia, arsine, and others can be found in acetylene produced from calcium carbide.
- All contaminants except phosphine and arsine are absorbed by passing the generated gas through water.
- Phosphine and arsine are removed from the gas by passing it through an acidified K2Cr2O7 or CuSO4 solution.
- Because acetylene is almost insoluble in water, the pure gas obtained is collected by downward displacement of water.
Uses of Acetylene
Lets look at some interesting uses of Acetylene.
- Acetylene is a useful molecule that can be converted into a wide range of compounds. Acetylene interacts with water vapor to create acetic acid and vinyl chloride in the presence of an acidic catalyst.
- Because of the high temperature of acetylene flames (33000C), acetylene is commonly used in welding procedures in many industries. This flame is used as incandescent illumination in some less developed countries.
- Acetylene is utilized in the production of rubber. The gas is utilized in the vulcanization of rubber, which gives it strength and elasticity. Vulcanization is the process of heating and chemically treating rubber to make it stronger and more durable.
- Acetylene is utilized in the manufacture of a variety of medications.
- Acetylene is frequently used in the production of polymers and resins. Acetylene is a gas composed of two carbon atoms that is very reactive. When utilised in the production process, it produces polymers and resins that are extremely strong and long-lasting. This makes them suitable for a wide range of applications, including automobile parts, building materials, and medical devices.








 CUET UG Final Answer Key 2025 Revised, D...
CUET UG Final Answer Key 2025 Revised, D...
 DU Cut off 2025, Delhi University Expect...
DU Cut off 2025, Delhi University Expect...
 OUAT Result 2025 OUT @ouat.nic.in: Check...
OUAT Result 2025 OUT @ouat.nic.in: Check...









