Table of Contents
Surface Tension: The phenomenon referred to as surface tension is caused by the cohesive forces / binding forces between liquid molecules. Surface Tension happens when the surface of a liquid comes into touch with another phase, which can also be a liquid. The liquid’s surface functions like an elastic sheet. Also, Liquids prefer to have the smallest possible surface area. All neighboring molecules share the cohesive forces between molecules in a liquid. Molecules on the surface of liquid have no neighboring molecules above them and hence have larger attractive forces on those on and below the surface.
Surface Tension
What is Surface Tension? Surface tension is a feature of a liquid’s surface that permits it to resist an external force due to the cohesive qualities of the water molecules. Surface tension energy can be thought of as being roughly similar to the effort or energy necessary to remove the outermost layer of molecules in one area. Surface tension is determined not only by the forces of pull between particles inside a specific liquid, but also by the forces of attraction of solids, liquids, and gases in contact with it. In this article, we will know the reason of Surface tension, Surface tension, formula, unit, and examples in detail with practice questions.
Surface Tension Simple Definition
Let’s utilize the example of a glass of water to explain the concept of surface tension. Because molecules located at the surface of a glass of water lack additional water molecules on all sides of them, they cohere more strongly to those that are directly linked with them (in this instance, the water molecules next to and underneath them, but not above them). It is not accurate that a “shell” forms on the water’s surface; the stronger cohesion among the water molecules, in contrast to the attraction of the water molecules to the air, makes moving an object through the surface more difficult than moving it when entirely covered.
Surface Tension Examples
Why do tiny insects walk on water? why bubbles are also round? There are numerous examples of surface tension in nature. Following are some surface tension examples and their explanation.
- Small insects, such as the water strider, may walk on water since their weight is insufficient to penetrate the surface.
- Water’s surface tension will bridge gaps in the tent material, making it rainproof.
- Jaundice clinical test with the help of surface tension– The surface tension of normal urine is about 66 dynes/centimeter, but if bile is detected (a test for jaundice), it reduces to about 55. Powdered sulfur gets spread over the urine surface during the Hay test. It floats in normal pee but sinks when the surface tension is reduced by the bile.
- The bubbles are circular: Water’s surface tension provides the essential wall tension for the forming of bubbles. The propensity to reduce wall tension causes the bubbles to form spherical forms.
- Droplets and surface tension: The form of liquid droplets is determined by surface tension. Despite being easily disfigured droplets of water are drawn into a spherical shape by the surface layer’s cohesive forces.
- Disinfectants with low surface tension are known as surface tension disinfectants. This allows them to break out and damage bacterial cell walls.
- a needle being floated on the water’s surface is also an example of surface tension.
- Cleaning garments with soaps and detergents that reduce water surface tension
Surface Tension of Various Substances
The surface tension of water at various temperatures and the surface tension of other liquids at particular temperatures are listed below.
| Surface Tension of Various Substances | |
| Substance |
Surface Tension (N/m)
|
|
|
| Ethanol | 22 |
| Mercury (20°C) | 0.44 |
|
Sodium Chloride
|
114 |
| Hydrogen | 2.4 |
| Oxygen (-193°C) | 0.016 |
| Helium | 0.16 |
| Blood, whole (37°C) | 0.058 |
| Blood, plasma (37°C) | 0.073 |
| Benzene (20°C) | 0.029 |
| Soap solution (20°C) | 0.025 |
What causes Surface Tension?
The liquid particles are drawn together by forces between molecules such as the Van der Waals force. The particles are drawn towards the remaining portion of the liquid across the surface. Let us analyze the molecular basis of surface tension.
- Water, aside from mercury, has the highest surface tension of any liquid.Water molecules want to stick collectively. However, because there is air above (and thus no water molecules), there are fewer water molecules that adhere to near the surface.
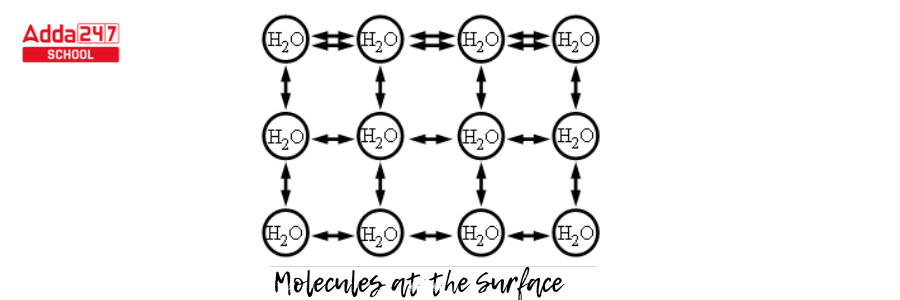
- As a consequence, the molecules that do come into contact with one another form a stronger bond, and a layer of highly bound water forms (see diagram).
- This surface layer, maintained together by surface tension, forms a significant barrier between the surrounding environment and the water.
- A molecule cannot provide a net force within its fluid body because the forces of the neighboring molecules cancel out (diagram given below). However, because there is no pulling force acting from above, a molecule on the liquid’s surface will experience a net inward force.
- Because of this inward net force, molecules that are on the surface compress and prevent being pushed out or broken. As a result, the surface is tense, which is possibly where the term “surface tension” originates from.
- Small items will “float” on the outermost surface of a fluid due to surface tension as long as they do not break through and split the uppermost layer of water molecules. When an object is placed on the fluid’s surface, the surface-bearing tension behaves like a membrane with elastic properties.
Surface Tension Formula
The proportion between the surface force (F) and the length (L) along which it acts is known as surface tension. Surface tension can be stated mathematically expressed as follows:
Surface Tension Formula –
Where,
F stands for force per unit length.
L denotes the length over which force acts.
T denotes the liquid’s surface tension.
Surface Tension Unit
Surface Tension: The surface tension of a liquid tends to reduce its surface area. Cohesive forces between liquid molecules cause surface tension. Surface tension is expressed mathematically as the force (F) exerted per unit length of surface (L).
T = F/L
- Because surface tension is force per unit length, its unit will be the unit of force/unit of length = Newton/meter. The unit of force is Newton, and the unit of length is meter. As a result, the unit of surface tension is Newton/meter.
- Surface Tension SI Unit – N/m
- Surface Tension CGS Unit – dyn/cm
Surface Tension Dimensional Formula
Surface tension, as we know, is determined by the formula Surface tension T = F/L.
We know that F = ma, so we can substitute the value in the equation to obtain =ma/L.
We derive =MLT-2L-1 by plugging the fundamental quantities into the equation.
Further investigation yields =MT-2.
As a result, the surface tension dimensional formula is MT-2.
Surface Tension Practice Problem
Here’s a surface tension practice problem given to know how to use the formula to calculate surface tension.
Question: Calculate the surface tension of a particular liquid with a pulling force of 9 N and a length of 2 m in which the force acts.
Solution: Given,
F = 9 N L = 2 m
The formula states that
T = F/L
⇒ T = 9/2
⇒ T = 4.5 N/m


 NIT Delhi Cutoff 2024 Out, Check Categor...
NIT Delhi Cutoff 2024 Out, Check Categor...
 Gujarat Ayurved University Result 2024 O...
Gujarat Ayurved University Result 2024 O...
 SOF IMO Cut Off 2024-25, Check Previous ...
SOF IMO Cut Off 2024-25, Check Previous ...































