Energy is a basic term in chemistry that governs the interactions and changes that characterize the cosmos. The First Law of Thermodynamics, which stipulates the conservation of energy in an isolated system, is a cornerstone of the principles governing energy. This article will explore the nuances of the First Law, shedding light on its significant applications and implications. The detailed PDF notes on the chapter on thermodynamics is also given which will prepare students for the NEET exam.
Explain First Law of Thermodynamics
The first law of thermodynamics is a big thinker. The first law of thermodynamics declares that the total amount of energy does not change. In other words, energy cannot be generated or destroyed, according to the First Law of Thermodynamics. It can only be transferred from one form to another. This concept may appear abstract at first, but if we look at examples, we’ll notice that energy transfers and transformations occur all the time around us. For instance:
- In Light bulbs, the conversion of electrical energy to light energy occurred.
- One pool ball collides with another, transferring kinetic energy and causing the second ball to move.
- Plants transform the energy of sunlight (radiant energy) into chemical energy that is stored in organic molecules.
- When any living organism moves, breathe, walk, by these actions they are converting chemical energy(Which is created by the chemical reaction in their body) into kinetic energy
First Law of Thermodynamics Equation
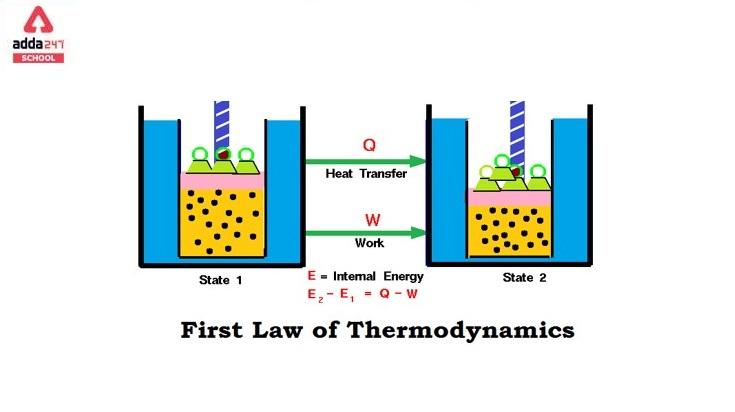
When a specific amount of heat is applied to a system, the amount of heat absorbed by the system is equal to the total increase in internal energy of the system and the external work done by the system, according to this law. Alternatively, the change in internal energy equals the difference between the total heat given to the system and the work done by the system. Let’s understand by the First law of thermodynamics equation given below:
ΔU = ΔQ – ΔW
Where,
ΔU = It depicts the change in Internal energy
ΔQ = It depicts Heat given to the system
ΔW = It depicts Work done by the system.
Hence, the heat given to the system is:
ΔQ = ΔU + ΔW
The first law of thermodynamics describes how the heat supplied to a system is connected to its internal energy and work done. The majority of useful inventions, such as heat engines, refrigerators, and air conditioners, are founded on this law.
Steam Engine: To understand the first law of thermodynamics, consider a steam engine. The mechanism of the steam engine is to convert heat energy into mechanical energy. The water is heated, which produces steam, which creates pressure to drive the piston within a cylinder, which propels the engine. As a result, when one type of energy disappears, an equal amount of another type of energy arises. It is impossible to construct a machine that can be run without energy.
First Law of Thermodynamics Example
It refers to a region of the physical cosmos with a specified limit that is being observed. This system can undergo internal modifications and exchange energy or mass with its surroundings. The system’s boundary divides it from its surroundings.
Open System in Thermodynamics
In an open system, both mass and energy are transferred between the system and its surroundings. It denotes the possibility of energy or mass leaving or entering the system. A swimming pool, for example, is an open system since water can enter and exit it.
Closed System in Thermodynamics
Only energy is transferred in a closed system; no mass is transferred. As an
example, consider a closed cylinder. It can be heated or cooled without losing bulk. However, if the cylinder is opened, the system behaves as if it were open.
Isolated System in Thermodynamics
There is no exchange of energy or mass with the external environment in an isolated system. Hot tea in a thermos flask or an insulated vessel, for example, is an isolated system since neither energy nor mass exchanges with the surroundings.
First Law of Thermodynamics Deals with only
- The first law of thermodynamics has a drawback in that it says nothing about the direction of heat flow.
- It makes no distinction between whether the procedure is spontaneous or not.
- It is not possible to reverse the process. You can not alter the first law of thermodynamics, i.e. heat can not be converted into work. If it had been possible to transform all of the heat into work, we could propel ships across the ocean by extracting heat from the water.
The First Law of Thermodynamics is a special case of this process
Isothermal process: During an isothermal operation, the temperature of an ideal gas remains constant. This signifies that the system’s heat is being used to conduct work against the environment. So,
dQ = dU + dW
dQ = dW (Where, dU = 0)
Melting process: When a solid melts into a liquid, the internal energy of the solid increases.
Amount of heat that the system absorbs = dQ
dV = 0 (Volume will be unaffected, hence change in volume will be zero)
dQ = dU + dW
dW = 0
dQ = dU
It demonstrates that the heat delivered is equivalent to the increase in the system’s internal energy.
Thermodynamics Chemistry NEET Notes PDF
Thermodynamics is one of the few concepts of science that is common in both the physics as well as chemistry domain. So covering this chapter is going to help students in both physics as well as the chemistry section of NEET exam. The importance of this chapter can be analyzed from this point that every year, there is at least 1 question in the chemistry section of the NEET exam from this chapter. Mastering this chapter will not only help students gain more marks in the NEET exam, but will also help in understanding other concepts of the NEET chemistry syllabus. To make the learning journey easier for students, our expert faculty at Adda 247 have created a detailed notes on the chemistry chapter that covers all the important concepts related to this chapter. Along with this, previous year questions of the NEET chemistry exam on this topic with their solutions is also given in the PDF. Students must go through the PDF given below for getting a complete understanding on this topic.
Download Thermodynamics Chemistry Notes PDF for NEET
Thermodynamics Chemistry Video Lecture for NEET
The detailed video lecture on the topic of thermodynamics have been created by one of nation’s best chemistry faculty for NEET exam. By going through the video lecture, students will have all their doubts resolved.
Read More About:
- Uric Acid: Normal Range, Medicine, Control, Treatment
- Aerobic Respiration
- Five Sense Organs For Kids Of Class 1 And 2
- Glycolysis: Pathway, Reaction, Diagram
- Viscosity Of Water: Meaning, Formula, Unit, Examples, Symbol
- Omnivore Animals









 Jawahar Navodaya Vidyalaya Admission For...
Jawahar Navodaya Vidyalaya Admission For...
 Karnataka DCET Seat Allotment Result 202...
Karnataka DCET Seat Allotment Result 202...
 AP POLYCET Seat Allotment Result 2025 OU...
AP POLYCET Seat Allotment Result 2025 OU...









