Benzene Structure: The benzene structure is cyclic, comprising six carbon atoms and six hydrogen atoms. Each carbon atom in benzene is linked to one hydrogen atom and two additional carbon atoms. Benzene has the chemical formula C6H6. It is found in a variety of plants and animals, and it is also produced by volcanic eruptions and forest fires. Benzene is the primary compound of a large number of aromatic compounds. In addition, benzene is utilized in the chemical industry to produce coal and oil. Benzene is also combined with other chemicals to make a variety of products such as plastic, detergent, rubber, and so on.
Benzene Structure
In the year 1825, Michael Faraday synthesized benzene by utilizing an illuminating gas. In 1834, a German chemist fused benzoic acid with lime to generate benzene. When they begin to heat both, the best outcomes are obtained in which benzene is created. In 1845, A.W. von Hofmann, a German chemist, extracted benzene from coal tar. Although benzene is a naturally occurring material created by eruptions and forest fires and found in many plants and animals, it is also a key industrial chemical derived from coal and oil. Scroll down to get comprehensive details about Benzene structure, Formula, resonance, properties, preparation, and uses.
Benzene Structure Formula
C6H6 is the formula for benzene, an aromatic organic compound. The presence of benzene can be detected by its distinctive odor. It is a poisonous, flammable liquid that is produced as a byproduct of coal distillation and is utilized as an industrial solvent. Understand the benzene structure here.
- Benzene has six hydrogen atoms and six carbon atoms, and its molecular weight is 78.11g/mol.
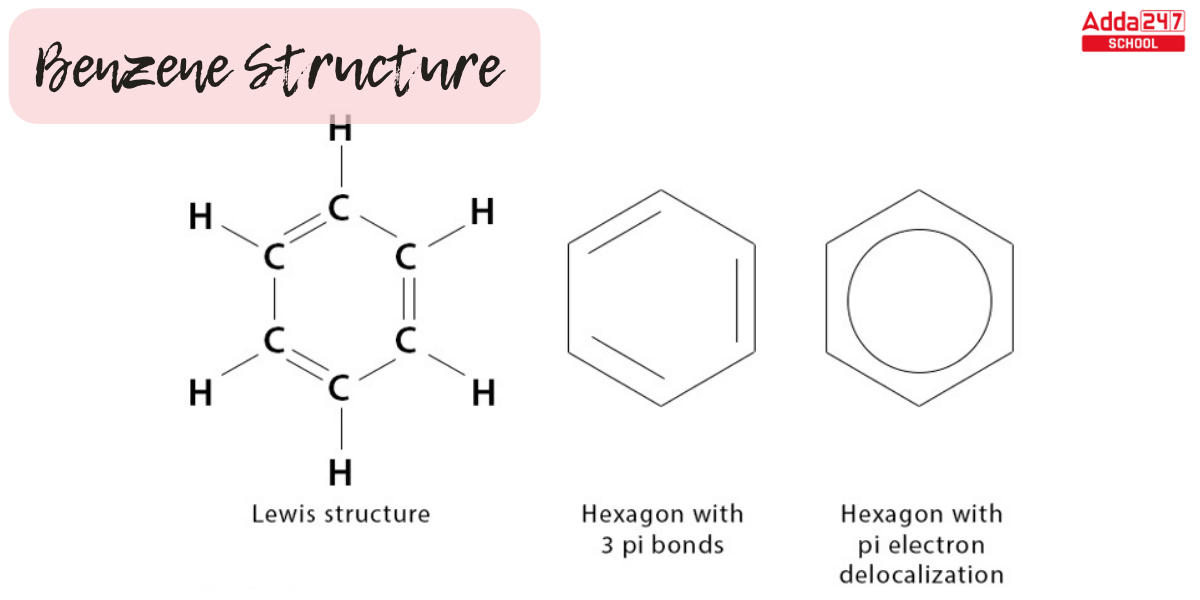
- The benzene structure seen above comprises six carbon atoms in a hexagon ring with alternate double bonds.
- The double bonds are known as conjugated double bonds, and a circle is used to symbolize six pi electrons inside the hexagon.
- Each carbon atom gives one of its two 2p electrons to form a delocalized pi system in benzene, a six-carbon aromatic annulene.
- Benzene is a planar molecule with three delocalized electrons above and below the plane.
- Electron delocalization makes the benzene ring more stable, and the bond length of all CC bonds is 1.47A with a bond angle of 120.
Benzene Structure Diagram
Benzene appears as a colorless liquid with a distinct scent. The 2D Benzene Structure Diagram model and Benzene lewis structure are shown below.
Benzene Structure Discovery
- As previously noted, benzene was found in 1825 in illuminating gas by scientist Michael Faraday.
- Eilhardt Mitscherlich, a German chemist, created benzene by heating benzoic acid with lime in 1834.
- A.W. von Hofmann, a German chemist, extracted benzene from coal tar in 1845.
- Since the finding, the benzene structure has piqued scientists’ interest. In 1861 and 1866, German chemists Joseph Loschmidt and August Kekule von Stradonitz suggested a cyclic structure of six carbons with alternating single and double bonds.
- Kekule then changed his structural formula so that the oscillation of the double bonds produced two equivalent structures in quick equilibrium.
- Linus Pauling, an American chemist, proposed in 1931 that benzene had a single structure that was a resonance hybrid of the two Kekule structures.
Preparation of Benzene
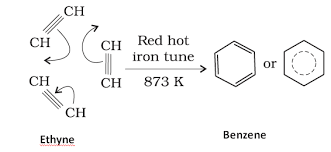
- Preparation of Benzene from Alkynes –
Benzene is synthesized from alkynes through cyclic polymerization of ethyne. Ethyne is run through a red-hot iron tube at 873 K in this procedure. The ethyne molecule is then cyclically polymerized to generate benzene.
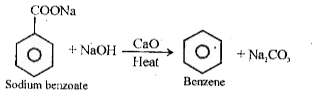
- Decarboxylation of aromatic acids –
Benzene is produced by heating sodium benzoate with sodium hydroxide in the presence of calcium oxide.
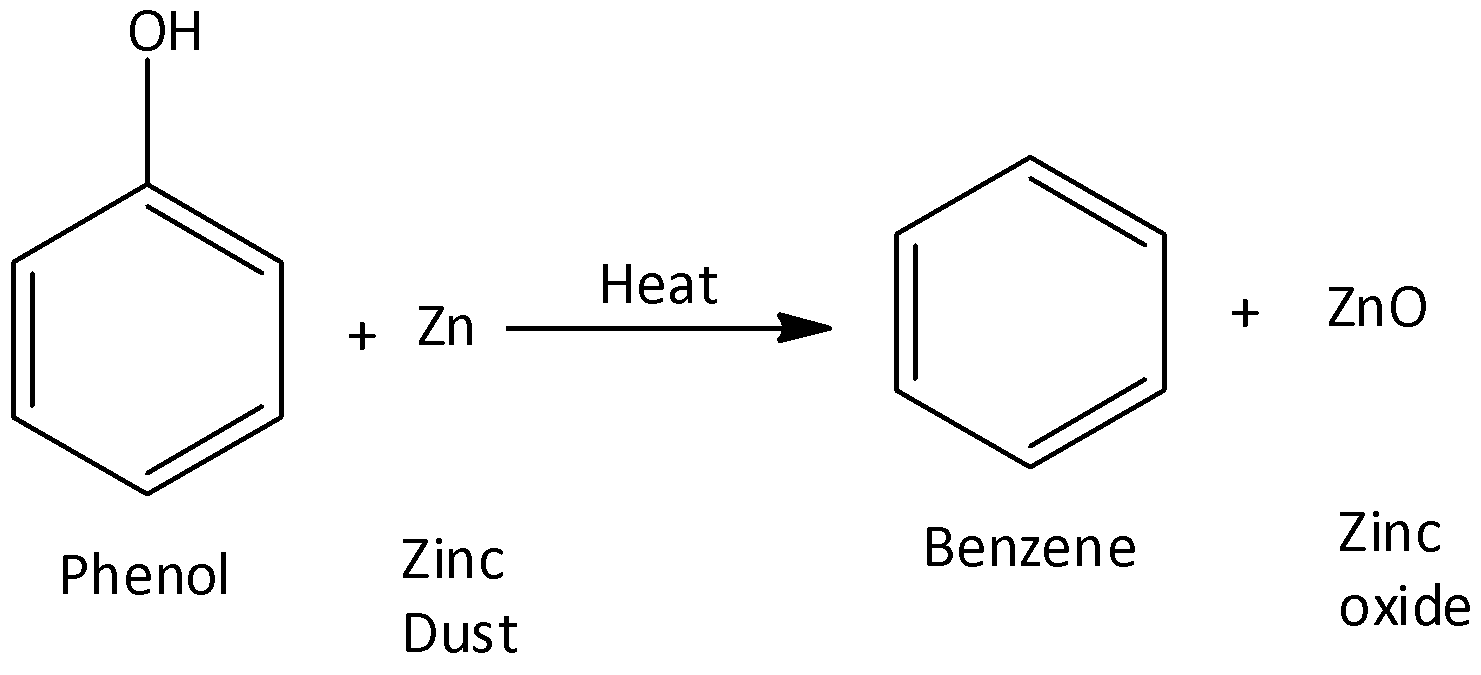
- Benzene synthesis from phenol
Benzene can also be made from phenols by reducing them. In this procedure, phenol vapors are passed through heated zinc dust. Zinc dust converts them to benzene.
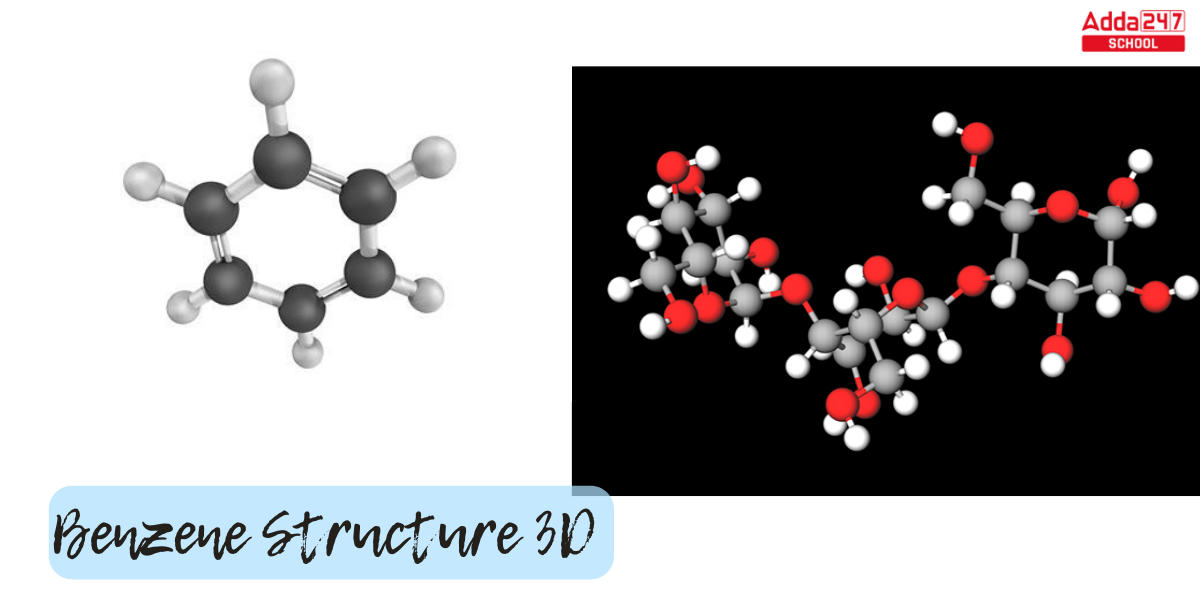
Benzene Structure 3D
The Benzene Structure 3d structure is represented below.
Benzene Structure Resonance
What is Resonance? A resonance form is another way to express a molecule’s Lewis dot structure. Resonance structures develop when there are various methods to construct a Lewis dot diagram that fulfills the octet rule (the octet rule refers to the mechanism by which an atom earns, loses, or shares electrons in order to have an outer electron shell with eight electrons).
- In benzene, Kekule proposed two cyclohexatriene Kekule structures that, when combined, form the general structure as contributing structures.
- The oscillating double bond that creates a ring structure in Benzene (C6H6) was well explained using resonance structure based on the balance bond theory. The carbon atom in the benzene ring produces sp2 hybridization.
- The two sp2 hybridized orbitals of an atom merge with the sp2 orbital of a nearby carbon atom to make 6 carbon-carbon bonds, while the remaining SP2 hybridized orbital combines with hydrogen orbitals to form 6 carbon Hydrogen bonds.
- Furthermore, another orbital, an unhybridized p orbital of carbon atoms, will form pi bonds with nearby carbon atoms via lateral overlapping. The benzene atom bonds are C1-C2, C3-C4, C5-C6, or C2-C3, C4-C5, C6-C1 bonds.
Benzene Physical Properties
Benzene is a non-polar solvent, a carcinogen, and an environmental hazard. It is an aromatic annulene, a volatile chemical molecule that belongs to the benzene family. The following section discusses the physical properties of benzene.
- It has a physical character that is liquid.
- Benzene has no colour.
- It has a melting point of 5.5 and a boiling point of 80.1 degrees Celsius.
- It is insoluble in water but soluble in polar solutions.
- It does have a pleasant aroma.
- It has a density of 0.87 g/cm3 and weighs less than water.
- Resonance exists in benzene.
- it is flammable.
Benzene Chemical Properties
Benzene is a chemical compound that appears frequently in Organic Chemistry. It has a structure of resonance. Benzene’s chemical characteristics are detailed more below.
- Nitration- At 55°C, benzene combines with nitric acid in the presence of sulfuric acid to generate nitrobenzene.
- Sulphonation is the process by which benzene is heated with fuming sulfuric acid to form benzene sulfonic acid.
- Friedel Craft’s Alkylation Reaction- When benzene is treated with an alkyl halide in the presence of Lewis acid, such as anhydrous aluminum chloride, alkylbenzene is formed. The Friedel craft alkylation reaction is the name given to this reaction.
- Halogenation occurs when benzene combines with halogen in the presence of Lewis acid to generate aryl halide.
- Friedel Craft’s Acylation Reaction- When benzene is treated with an acyl halide in the presence of Lewis acid, it produces acyl benzene.
Benzene Structure & Uses
Benzene is an organic and simple aromatic hydrocarbon that is the parent compound of a large variety of significant aromatic compounds.
- This chemical is primarily used in the production of polystyrene.
- It is used in the manufacture of phenol.
- It is utilized in the production of nylon fibers.
- It is employed as an organic compound solvent in the synthesis of numerous valuable chemicals.
Benzene Health Effects
In the body, benzene degrades into various different chemicals.
- These chemicals can be identified in the blood or urine of people who have recently been exposed to high quantities of benzene.
- Long-term benzene exposure can raise the risk of acquiring leukemia.
- Benzene exposure can cause anemia and damage the immune system.
- According to animal research, breathing benzene vapors can harm reproductive organs and cause sterility. Menstrual irregularities have been linked to benzene exposure in the workplace.








 CUET UG Final Answer Key 2025 Revised, D...
CUET UG Final Answer Key 2025 Revised, D...
 DU Cut off 2025, Delhi University Expect...
DU Cut off 2025, Delhi University Expect...
 OUAT Result 2025 OUT @ouat.nic.in: Check...
OUAT Result 2025 OUT @ouat.nic.in: Check...









