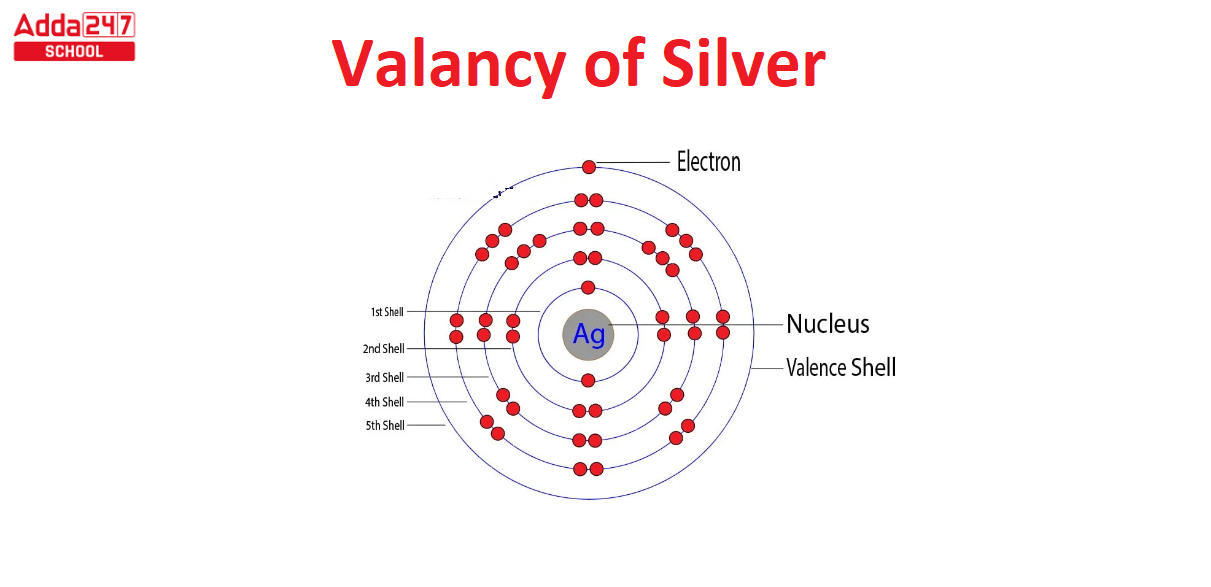
All, chemistry enthusiasts Students! So, you’ve heard about the valency of elements like hydrogen, oxygen, and carbon. But what about the star of today’s blog is silver? We often associate silver with beautiful jewelry or valuable coins, but there’s a whole scientific angle you might not know about. In this post, we’ll dive deep into the world of silver’s valency—yes, it’s a thing. Stick around to discover what sets silver apart from its shiny sibling, gold, and why understanding silver’s valency matters in compounds like AG2S and AGNO3. Ready to go treasure hunting through chemistry? Let’s dig in!
What is Valency?
Before we jump into the heart of the matter, let’s quickly demystify the term “valency.” In layman’s terms, valency refers to how many electrons an atom is willing to share, lose, or gain to form a bond with another atom. Simple enough, right? In the case of silver, though, things get a tad more interesting.
Valency of Silver Ion
Silver ions usually have a valency of +1. This means a silver ion likes to lose one electron to get that stable, happily-ever-after state. Silver isn’t the social butterfly like oxygen, which forms multiple bonds. Nope, silver is more of a lone wolf, sticking to a single bond most of the time.
Valency of Silver and Gold: A Side-By-Side Comparison
You might be wondering, “Hey, how does silver compare to gold in terms of valency?” Good question! Like silver, gold also commonly has a valency of +1. However, gold can occasionally show off and have a valency of +3. Silver, being the modest element that it is, usually sticks to its +1 valency.
Silver Valancy in Specific Compounds
Ready for some chemistry acrobatics? Let’s explore how silver behaves in particular compounds.
Valancy of Silver in AG2S
In silver sulfide (AG2S), each silver atom has a valency of +1. You get two silver atoms (Ag) each sharing their lonely electron with a single sulfur (S) atom. It’s like a chemical ménage à trois!
Valancy of Silver in AGNO3
Silver nitrate (AGNO3) is another interesting compound. Here, silver also has a valency of +1. It’s bonding with the nitrate ion (NO3), which carries a -1 charge. They’re like the perfect match on a dating app—equally charged and ready to bond!
Valancy of Silver Nitrate
Still curious about silver nitrate? Well, apart from being used in photography and medical treatments, it’s a prime example of silver’s +1 valency in action.
Valancy of Silver Chloride
Ever heard of silver chloride (AgCl)? In this compound, silver also maintains a +1 valency. It’s commonly used in photographic paper and is another poster child for silver’s straightforward bonding behavior.
Check: Valency of Zinc
Valency of Silver- Why Does It Matter?
Understanding the valency of silver can help us in various applications—from creating alloys and chemical solutions to understanding its behavior in biological systems. Plus, it’s always good to know what’s behind the bling, right?
Silver Valency Conclusion Facts
So there you have it! Silver mostly has a valency of +1, and it’s quite the introvert when it comes to forming bonds. Whether it’s comparing it with gold or understanding its role in compounds like AG2S or AGNO3, silver’s valency is a fascinating topic for both budding and seasoned chemists.








 Electrochemical Series: Meaning, Table, ...
Electrochemical Series: Meaning, Table, ...
 Difference Between Acid and Base, Know B...
Difference Between Acid and Base, Know B...
 Solution Class 12 Notes for NEET, Downlo...
Solution Class 12 Notes for NEET, Downlo...









