The fascinating scientific field of electrochemistry deals with chemical processes involving the exchange of electrons between electrodes. These reactions, which are also known as redox reactions, provide the basis for a number of phenomena, including the rusting of metal and the production of energy in batteries. Electrochemistry is present in many facets of our lives, from the batteries that run our electronics to the electrolysis procedures that are essential to industry. The chapter is highly important when it comes to the board exams and competitive exams. Due to its high significance, every year, many questions are asked in the exam on this topic. So, preparing this topic from the right resource becomes extremely important. For this purpose, we have provided students with the precious notes pdf of this topic that will not only cover the board syllabus but also the NEET exam syllabus.
Electrochemistry
The study of the correlation between electrical energy and chemical changes in an electrochemical cell is known as electrochemistry. From the single-use batteries in our remote controls to the lithium-ion batteries in our phones, we come into contact with electrochemical cells in all aspects of our daily lives. Electrochemical reactions are chemical processes that involve the input or creation of electric currents. These reactions involve electrons moving between electrodes divided by an ionically conducting and electronically covering electrolyte (or ionic species in a solution) via an electronically-conducting phase (typically an external electrical circuit, but not always, as in electroless plating).
Electrochemistry: What is an Electrochemical reaction?
An electrochemical reaction occurs when a chemical reaction is triggered by an electrical potential difference, as in electrolysis, or when a potential difference comes from a chemical reaction, as in an electric battery or fuel cell. Such reactions are roughly grouped into two categories: the production of chemical change by electrical energy, known as electrolysis, and the conversion of chemical energy into electrical energy, known as spontaneous redox reactions. Unlike other chemical reactions, electrons in electrochemical reactions are transmitted via an electronically-conducting circuit rather than directly between atoms, ions, or molecules.
Electrochemistry: Oxidation & Reduction
Electrochemistry often deals with the overall reactions that occur when numerous redox reactions occur concurrently, coupled by a particular outside electric current and a suitable electrolyte. In other terms, electrochemistry is concerned with chemical events involving charge separation (as observed frequently in liquids like solutions). The term “redox” refers to the reduction-oxidation process. It refers to electrochemical processes that involve the transfer of electrons to or from a molecule or ion to change its oxidation state. This reaction can be triggered by an external voltage or by the release of chemical energy. The terms oxidation and reduction refer to the changes in oxidation states that occur in the atoms, ions, or molecules involved in an electrochemical reaction.
Oxidation & Reduction Reaction Examples
- When atomic sodium combines with atomic chlorine, sodium gives one electron and achieves an oxidation state of +1. Chlorine accepts the electron, reducing its oxidation state to 1. The sign of the oxidation state (positive/negative) correlates to the value of the electronic charge of each ion. The attraction of the oppositely charged sodium and chlorine ions causes them to form an ionic bond.
- Placing a piece of copper wire in an aqueous solution of the Ag+ ion is a classic example of oxidation-reduction reactions. The process involves the net transfer of electrons from copper metal to Ag+ ions, resulting in silver whiskers growing out of the copper wire and Cu2+ ions.
Cu(s) + 2 Ag+(aq) = Cu2+(aq) + 2 Ag(s).
The Cu2+ ions generated in this reaction are responsible for the solution’s light-blue colour. The presence of these ions can be confirmed by adding ammonia to this solution, which produces the deep-blue Cu(NH3)42+ complex ion.
- magnesium metal and oxygen reaction –
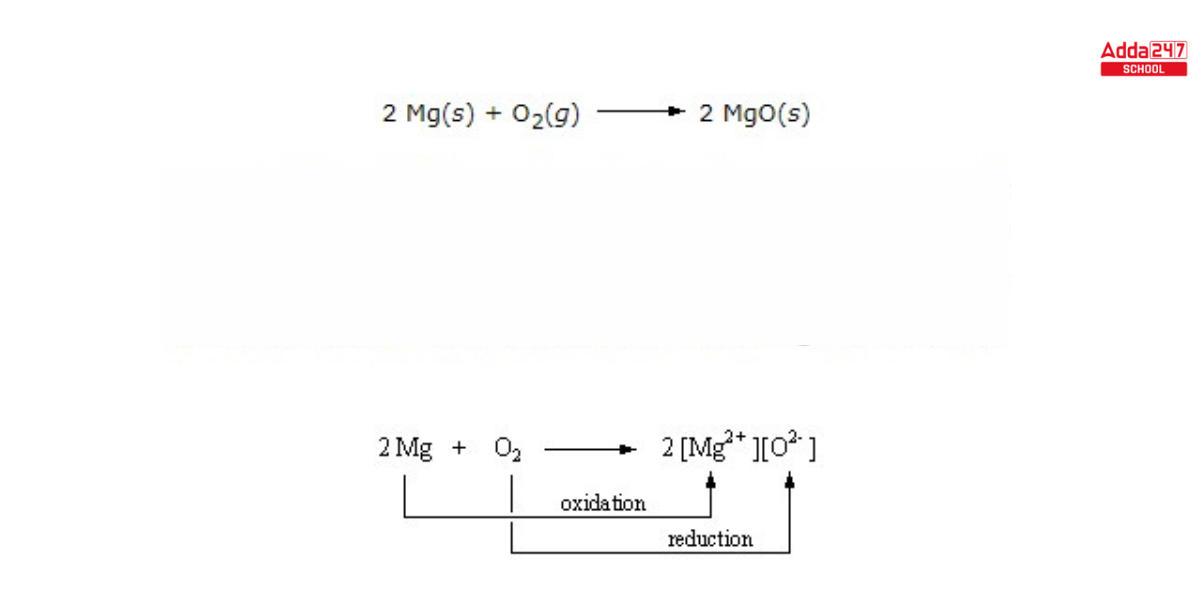
Electrochemical Cells
The Gibbs energy of the system decreases when a spontaneous chemical reaction takes place on its own in electrochemical cells. This energy is subsequently converted into electricity. It is also possible to induce non-spontaneous processes by supplying external energy in the form of electrical energy. Electrochemical cells are classified into two types: galvanic (also known as Voltaic) and electrolytic.
Galvanic cells get their energy from spontaneous redox reactions, whereas electrolytic cells require an external electron source, such as a DC battery or an AC power source. Both galvanic and electrolytic cells will have two electrodes -an anode and a cathode constructed of the same or different metals, as well as an electrolyte in which the two electrodes are immersed.
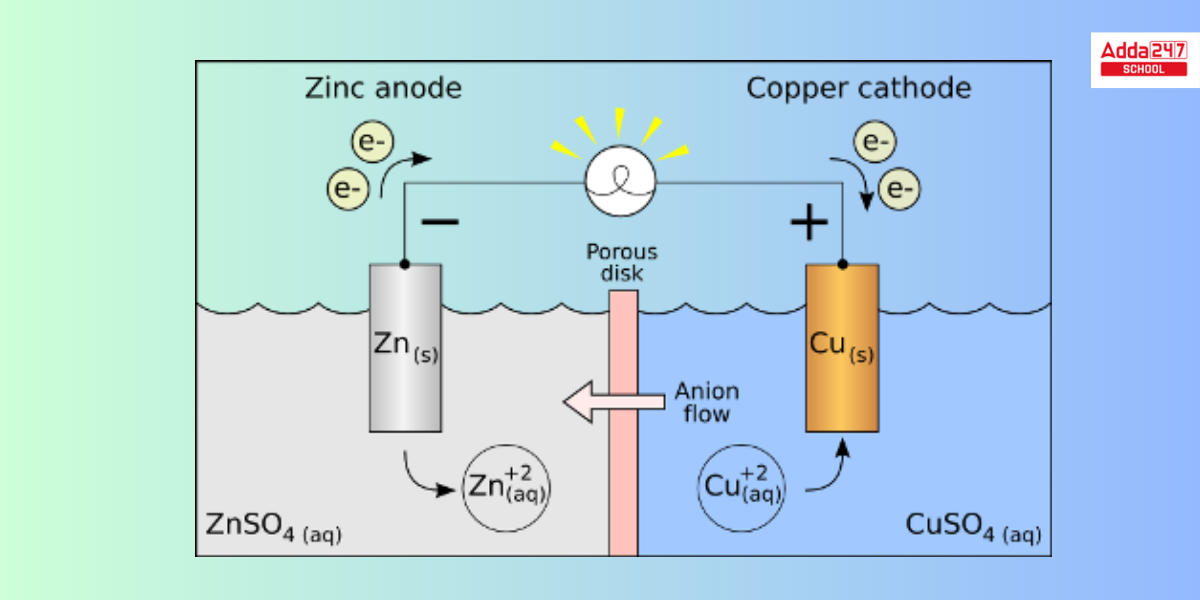
Galvanic Cell
The galvanic cell turns chemical energy into electrical energy, which is obtained through a redox reaction. Galvanic cells have traditionally been employed as DC power sources. Both oxidation and reduction occur in different compartments. Each compartment contains an electrolyte solution as well as a metallic conductor that serves as an electrode. Half cells are the compartments that contain the electrode and the electrolyte solution.
- Example – A Daniel Cell, for example, is a Galvanic Cell in which the redox reaction is carried out with Zinc and Copper. Zn is the reducing agent, and Cu2+ is the oxidizing agent.
Zn(s)+Cu2+(aq)→Zn2+(aq)+Cu(s)
Oxidation Half/ Anode: Zn(s)→Zn2+(aq)+2e−
Reduction Half/ Cathode: Cu2+(aq)+2e−→Cu(s)
Electrolytic Cell
A usually non-spontaneous reaction is driven by energy from an external power source in an electrolytic cell, which is an electrochemical cell. Unlike a galvanic cell, the electrodes of an electrolytic cell can be positioned in a single compartment that contains the molten or aqueous electrolyte. The electrodes are immersed in an electrolytic solution containing cations and anions in this step. When current is applied, the ions migrate towards electrodes of opposite polarity, where they undergo simultaneous reduction and oxidation. Electrolytic cells are encountered throughout the charging process of any rechargeable battery, from lead-acid batteries in automobiles to lithium-ion batteries in smartphones.
- Example – The electrolysis of molten sodium chloride involves melting sodium chloride (over 801oC), inserting two electrodes into the melt, and passing an electric current through the molten salt. The following chemical reaction occurs at the electrodes: Sodium ion migrates to the cathode, where it gains one electron and is reduced to sodium metal.
Na+ + e-→ Na Chloride ions travel to the anode, where they lose one electron and are converted to chlorine gas.
Cl-→1/2 Cl2 + e-The overall reaction is the dissolution of sodium chloride into its constituents – 2NaCl→ 2Na(s) + Cl2(g).
Electrochemistry Notes for NEET PDF
Electrochemistry is an crucial topic of the physical chemistry branch of the chemistry. This chapter is highly important for the chemistry section of the NEET exam. Each year, around 2 to 3 questions are asked in the NEET exam from this concept. This accounts for around 4 to 6 percent of the total question in the NEET chemistry section. Having a proper understanding of this topic will help students in earning the crucial 8-12 marks. It will help students get more close to getting admitted into their dream college. If studied from the right resources, this chapter becomes too much scoring due to its analytical nature. For the convenience of students, our expert chemistry faculty of NEET at Adda 247 have created a detailed notes on this topic in PDF form. The notes cover all the important formulas and concepts that will aid students in understanding this topic clearly. The PDF notes as per the lates NEET syllabus is given below for download.
Electrochemistry Notes PDF for NEET
Electrochemistry NEET Questions with Answers PDF
After learning the concept, it is always advised to solve some related questions and previous year questions. It helps students understand how to apply the learned concepts to solve problems related to that particular topic. So, to master the electrochemistry chapter fully for the NEET exam, it is important to solve previous year questions. It helps students to know about the pattern and difficulty level of questions asked in the exam. Solving previous year questions will also give a lot of confidence to students. For this, our expert faculty always stresses on solving as many previous year NEET questions as possible. To aid students in their preparation, NEET previous year questions with solutions pdf on electrochemistry is given below for free download.
Electrochemistry NEET Questions with Solutions PDF
Electrochemistry Video for NEET
The detailed video explanation on all the concepts of electrochemistry is given below for students. The video covers all the sub-topics of electrochemistry as per the latest NEET curriculum. The video lecture also contains NEET previous year questions to aware students about the type of questions asked in the NEET UG exam from this chapter. After going through these video lectures and revising the above-given notes PDF, students will become fully prepared for the electrochemistry part of the NEET syllabus.










 Try CUET College Predictor 2025 to Predi...
Try CUET College Predictor 2025 to Predi...
 CUET Result 2025 OUT (Today) @cuet.nta.n...
CUET Result 2025 OUT (Today) @cuet.nta.n...
 Why the Delay in CUET UG 2025 Results? C...
Why the Delay in CUET UG 2025 Results? C...









