Rutherford Atomic Model
The Rutherford model, also known as the Rutherford atomic model, nuclear atom, or planetary model of the atom, is a description of atom structure developed by Ernest Rutherford, a New Zealand-born scientist, in 1911. The model depicted the atom as a small, compact, positively charged centre called a nucleus, in which nearly all of the mass is concentrated, and around which the light, negative constituents known as electrons circle at a distance, similar to planets rotating around the Sun.
Rutherford Atomic Model in Hindi
रदरफोर्ड परमाणु मॉडल: रदरफोर्ड मॉडल, जिसे रदरफोर्ड परमाणु मॉडल, परमाणु परमाणु या परमाणु के ग्रहीय मॉडल के रूप में भी जाना जाता है, 1911 में न्यूजीलैंड में जन्मे वैज्ञानिक अर्नेस्ट रदरफोर्ड द्वारा विकसित परमाणु संरचना का विवरण है। मॉडल ने परमाणु को एक के रूप में दर्शाया है। छोटा, सघन, धनावेशित केंद्र जिसे नाभिक कहा जाता है, जिसमें लगभग सभी द्रव्यमान केंद्रित होते हैं, और जिसके चारों ओर प्रकाश, नकारात्मक घटक इलेक्ट्रॉन के रूप में जाने जाते हैं, जो सूर्य के चारों ओर घूमने वाले ग्रहों के समान दूरी पर होते हैं।
Rutherford Atomic Model: Diagram
Rutherford Atomic Model: Experiment for Class 9 and 11
The Rutherford Atomic Model experiment for Classes 9 and 11 are given below.
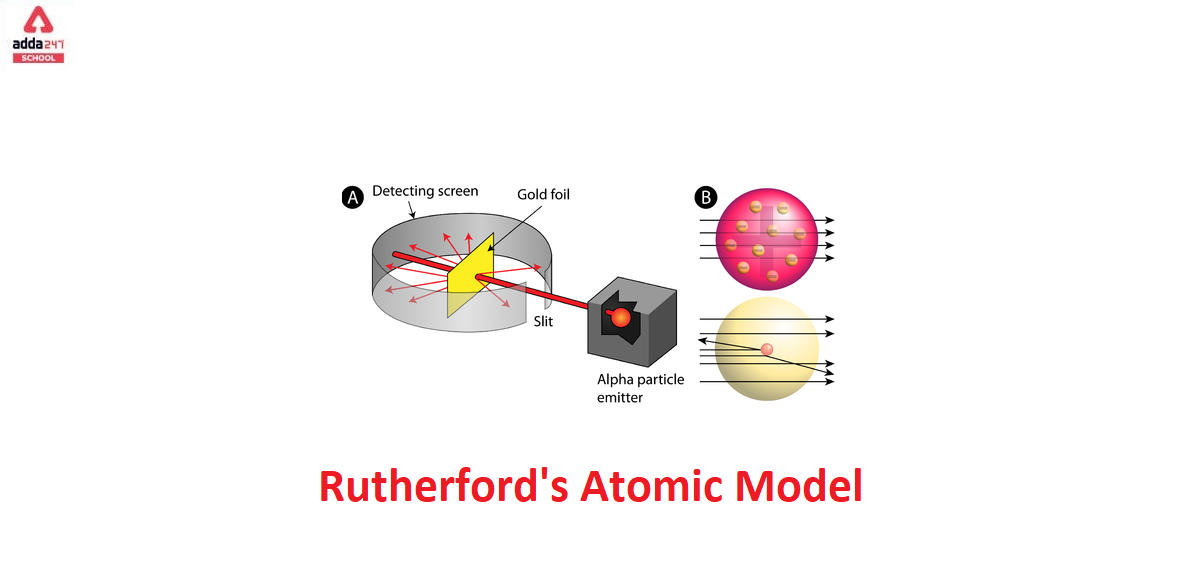
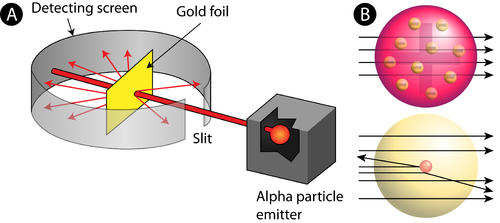
Rutherford Alpha Scattering Experiment
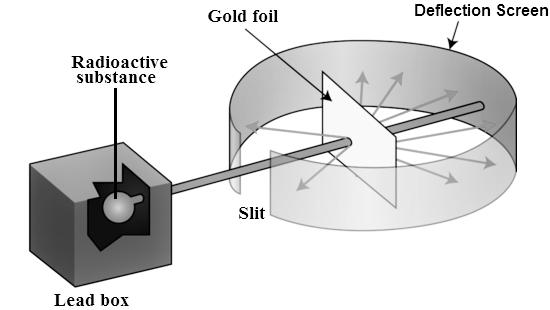
To account for the scattering of alpha particles from thin gold foil reported in a series of experiments conducted by undergraduate Ernest Marsden under the direction of Rutherford and German scientist Hans Geiger in 1909, the nucleus was proposed as small and dense. Within a protective lead shield, a radioactive source generating alpha particles (positively charged particles equivalent to the helium atom nucleus and 7,000 times heavier than electrons) was contained. After passing through a slit in a lead screen, the radiation was focussed into a narrow beam. To detect alpha particles, a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulphide to make it luminous acted as a counter.
Each alpha particle created a scintillation when it hit the fluorescent screen, which was seen through a viewing microscope mounted to the rear of the screen. Rutherford and his colleagues were able to move the screen around to see if any alpha particles were being deflected by the gold foil.
The vast majority of alpha particles slipped right through the gold foil, implying that atoms are largely made up of empty space. The tiny deflection of some alpha particles suggests interactions with other positively charged particles within the atom. Other alpha particles were thrown in all directions, and a few even bounced back toward the source. Such tremendous repulsion could only be explained by a positively charged and relatively hefty target particle, such as the suggested nucleus. Negative electrons were thought to circulate in circular orbits around the nucleus, balancing the positive nuclear charge electrically.
Rutherford Atomic Model: Year
Rutherford Atomic Model Experiment start in the year 1909 and was completed in 1911.
Rutherford Atomic Model: Postulates
Rutherford made the observations in his experiments and gave the following postulates:
- Positively charged particles and the majority of an atom’s mass were concentrated in a very small volume. This part of the atom was dubbed a nucleus by him.
- The Rutherford model stated that the nucleus of an atom is surrounded by negatively charged electrons. He also claimed that the electrons that surround the nucleus travel in circular routes at great speeds. These circular routes were given the name orbits by him.
- The nucleus is held together by a strong electrostatic force of attraction because electrons are negatively charged and the nucleus is a densely concentrated mass of positively charged particles.
Rutherford Atomic Model: Limitations
The Rutherford atomic theory was not some mere theory, but experiment-based observations. But, still, the theory had some shortcomings.
According to Rutherford, electrons orbit the nucleus in set trajectories termed orbits. According to Maxwell, accelerating charged particles release electromagnetic radiation, thus an electron rotating around the nucleus should do the same. This radiation would transmit energy from the electron’s motion, but at the expense of orbital shrinkage. The electrons would eventually collapse in the nucleus. According to calculations, an electron would disintegrate in the nucleus in less than 10-8 seconds using the Rutherford model. As a result, the Rutherford model contradicted Maxwell’s theory and was unable to explain atom stability.
Despite the fact that the early atomic models were erroneous and failed to explain certain experimental results, they served as the foundation for future quantum mechanics breakthroughs. One of the flaws of the Rutherford model was that he did not address the configuration of electrons in an atom, leaving his theory incomplete.
Rutherford Atomic Model Experiment in Hindi
रदरफोर्ड अल्फा स्कैटरिंग प्रयोग: 1909 में रदरफोर्ड और जर्मन वैज्ञानिक हैंस गीगर के निर्देशन में अंडरग्रेजुएट अर्नेस्ट मार्सडेन द्वारा किए गए प्रयोगों की एक श्रृंखला में रिपोर्ट की गई पतली सोने की पन्नी से अल्फा कणों के बिखरने के लिए, नाभिक को छोटे और घने के रूप में प्रस्तावित किया गया था। एक सुरक्षात्मक लीड शील्ड के भीतर, अल्फा कण उत्पन्न करने वाला एक रेडियोधर्मी स्रोत (हीलियम परमाणु नाभिक के बराबर धनात्मक आवेशित कण और इलेक्ट्रॉनों की तुलना में 7,000 गुना अधिक भारी) निहित था। एक लेड स्क्रीन में एक स्लिट से गुजरने के बाद, विकिरण को एक संकीर्ण बीम में केंद्रित किया गया था। अल्फा कणों का पता लगाने के लिए, सोने की पन्नी का एक पतला खंड स्लिट के सामने रखा गया था, और इसे चमकदार बनाने के लिए जिंक सल्फाइड के साथ लेपित एक स्क्रीन काउंटर के रूप में काम करती थी।
फ्लोरोसेंट स्क्रीन से टकराने पर प्रत्येक अल्फा कण ने एक जगमगाहट पैदा किया, जिसे स्क्रीन के पीछे लगे एक देखने वाले माइक्रोस्कोप के माध्यम से देखा गया। रदरफोर्ड और उनके सहयोगी यह देखने के लिए स्क्रीन को इधर-उधर घुमाने में सक्षम थे कि क्या कोई अल्फा कण सोने की पन्नी से विक्षेपित हो रहा है।
अधिकांश अल्फा कण सोने की पन्नी के माध्यम से सही फिसल गए, जिसका अर्थ है कि परमाणु बड़े पैमाने पर खाली जगह से बने होते हैं। कुछ अल्फा कणों का छोटा विक्षेपण परमाणु के भीतर अन्य सकारात्मक चार्ज कणों के साथ बातचीत का सुझाव देता है। अन्य अल्फा कण सभी दिशाओं में फेंके गए, और कुछ वापस स्रोत की ओर उछले। इस तरह के जबरदस्त प्रतिकर्षण को केवल एक सकारात्मक चार्ज और अपेक्षाकृत भारी लक्ष्य कण द्वारा समझाया जा सकता है, जैसे कि सुझाए गए नाभिक। ऐसा माना जाता था कि ऋणात्मक इलेक्ट्रॉन नाभिक के चारों ओर वृत्ताकार कक्षाओं में परिचालित होते हैं, जो विद्युत रूप से धनात्मक नाभिकीय आवेश को संतुलित करते हैं।
Rutherford Atomic Model is also known as
Rutherford model, also known as the Rutherford atomic model, nuclear atom, or planetary model of the atom, description of the structure of atoms discovered (1911) by the physicist Ernest Rutherford.








 BTEUP Result 2025 Out at bteup.ac.in, Do...
BTEUP Result 2025 Out at bteup.ac.in, Do...
 SGBAU Result 2025 Out, Check Summer Seme...
SGBAU Result 2025 Out, Check Summer Seme...
 How To Prepare for CUET Accountancy Exam...
How To Prepare for CUET Accountancy Exam...









