Maillard Reaction
The chemical reaction between amino acids and reducing sugars known as the Maillard process is what gives browned food its characteristic flavour. This response occurs in a variety of meals, including grilled steaks, fried dumplings, breads, cookies, and other types of biscuits. It was first described in 1912 by French chemist Louis Camille Maillard as he attempted to mimic the synthesis of biological proteins.
Maillard Reaction Temperature
Maillard is the name of the process. The process, which is a non-enzymatic browning reaction, normally moves quickly between 140 and 165 °C (280 and 330 °F). Several recipes specify an oven temperature that will guarantee the Maillard reaction takes place. Higher temperatures result in more pronounced caramelization and pyrolysis (the last breakdown that causes burning and the emergence of harsh tastes).
Maillard Reaction Mechanism
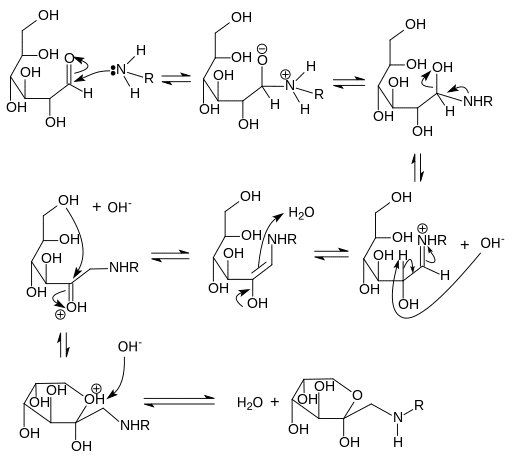
- N-substituted glycosylamine and water are produced when the carbonyl group of the sugar interacts with the amino group of the amino acid.
- Amadori rearrangement of the unstable glycosylamine results in the formation of ketosamines.
- The ketosamines can further react in a number of ways:
- Make two water molecules, as well as reductones.
- There can be formation of diacetyl, pyruvaldehyde, and other short-chain hydrolytic fission products.
- Make melanoidins and brown nitrogenous polymers.
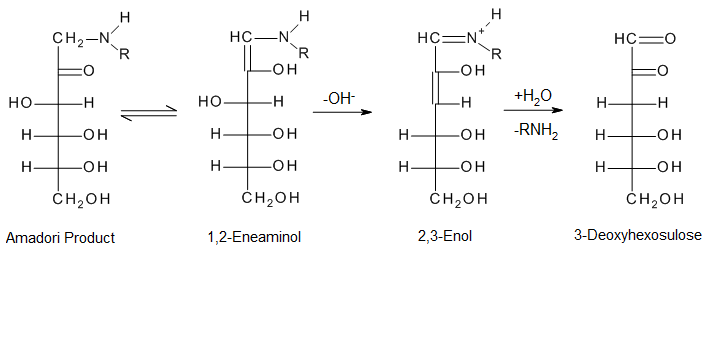
To create dicarbonyls, the open-chain Amadori products are further dehydrated and deaminated. This is an essential transition.
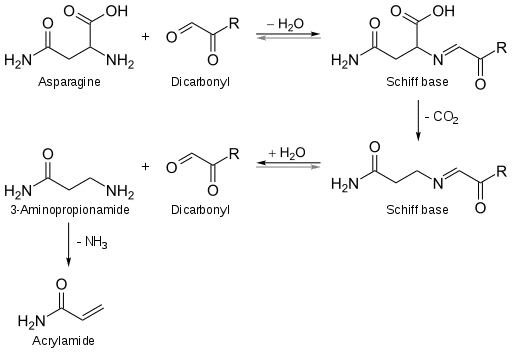
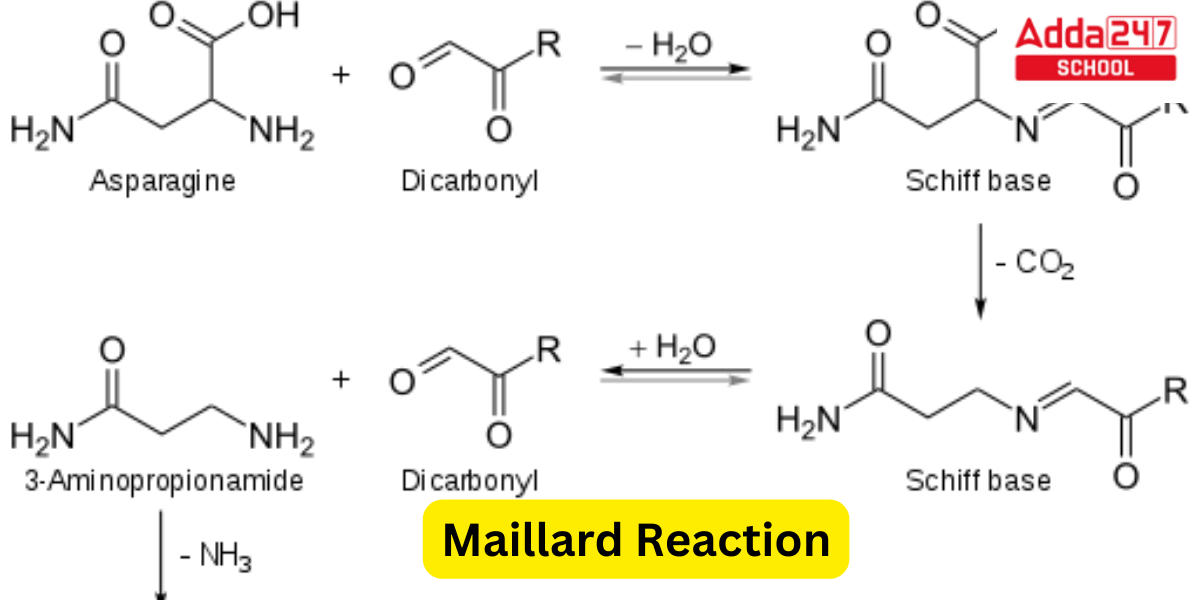
By Strecker degradation, dicarbonyls and amines form Strecker aldehydes. As a consequence of the Maillard reaction between reducing sugars and amino acids, particularly asparagine, both of which are present in most food products, acrylamide, a potential human carcinogen, can be produced.
Maillard Reaction: Examples
The browning of different meats when seared or grilled, the browning and umami flavour in fried onions, and coffee roasting are all results of the Maillard reaction. It affects the colour and flavour of dried and condensed milk, dulce de leche, toffee, black garlic, chocolate, toasted marshmallows, and roasted peanuts, as well as the darkened crust of baked goods, the golden-brown hue of French fries and other crisps, the browning of malted barley used to make malt whisky and beer.
- The biscuit- or cracker-like flavour found in baked foods like bread, popcorn, and tortilla products is caused by 6-acetyl-2,3,4,5-tetrahydropyridine.
- The scent and natural occurrence of the structurally related molecule 2-acetyl-1-pyrroline, which also arises without heating, are comparable.
- The substance is responsible for the distinctive aromas of many types of cooked rice and the herb pandan (Pandanus amaryllifolius).
- The odour thresholds for both substances are less than 0.06 nanograms per litre.
- It takes a variety of chemical events, including the breaking of the tetrapyrrole rings in the muscle protein myoglobin, to produce the complicated browning reactions that happen when meat is roasted or seared.
- With dried fruit, Maillard reactions also take place.
- Despite the fact that the results of the two processes can occasionally be identical to the naked eye, caramelization and Maillard browning are completely separate processes (and taste buds).
- Browning may occasionally result from caramelization in the same foods where the Maillard reaction takes place, but the two processes are separate.
- The Maillard reaction involves amino acids, whereas caramelization is the pyrolysis of specific sugars. They are both induced by heating.
- The Maillard reaction takes place throughout the silage-making process, which lowers the quantity of energy and protein accessible to the animals that consume it.
Maillard Reaction: Important Facts
- The nucleophilic amino group of the amino acid combines with the reactive carbonyl group of the sugar to generate a complex mixture of poorly understood compounds that are responsible for a variety of tastes and fragrances.
- The amino groups (RNH + 3 –> RNH2) are deprotonated in an alkaline environment, increasing their nucleophilicity and speeding up this process (for example, when lye is used to darken pretzels; see lye roll).
- Many of the formulations used in the flavouring industry are based on this response.
- A potential carcinogen known as acrylamide can develop at high temperatures.
- By heating at a lower temperature, using asparaginase, or introducing carbon dioxide, this can be prevented.
- Hundreds of distinct taste compounds can be produced during the cooking process by Maillard reactions, which are influenced by the chemical composition of the food, cooking temperature, cooking duration, and the presence of air.
- These chemicals frequently split apart to create new taste compounds.
- Over the years, flavour experts have created synthetic flavours using the Maillard process.
Louis Camille Maillard wrote about the interaction of amino acids and sugars at high temperatures in a work that was published in 1912. A mechanism for the Maillard reaction was developed in 1953 by chemist John E. Hodge working for the US Department of Agriculture.
Decomposition Reaction, Examples, Formula, Types, Definition For Class 10
Cannizzaro Reaction Mechanism, Definition, Example for Class 12
Endothermic Reaction Examples, Equations, Formula for Class 10








 CUET UG Final Answer Key 2025 Revised, D...
CUET UG Final Answer Key 2025 Revised, D...
 DU Cut off 2025, Delhi University Expect...
DU Cut off 2025, Delhi University Expect...
 OUAT Result 2025 OUT @ouat.nic.in: Check...
OUAT Result 2025 OUT @ouat.nic.in: Check...









