Endothermic Reaction
When a chemical link is broken, energy is normally released in the process. The creation of chemical bonds, too, necessitates an energy input. The energy that is supplied/released can take many different forms (such as heat, light, and electricity). Endothermic reactions are characterised by the creation of chemical bonds as a result of heat absorption from the environment. Exothermic reactions, on the other hand, involve the release of heat energy generated by bond-breakage.
What is Endothermic Reaction?
Endothermic reactions are chemical processes in which the reactants absorb heat from the environment to produce products. These reactions cause a cooling effect by lowering the temperature of the surrounding environment. Ice cubes absorb heat energy from their surroundings and melt to generate liquid water, which is an endothermic process.
Endothermic Reaction for Class 10 Students
A chemical process that is generally accompanied by the retention of warmth (or heat), or a living being that creates warmth (or heat) to maintain its temperature, is known as an endothermic reaction. A chemical process that operates only if heat is retained is an example of an endothermic process reaction. A warm-blooded animal, such as humans, is an example of an endothermic mammal.
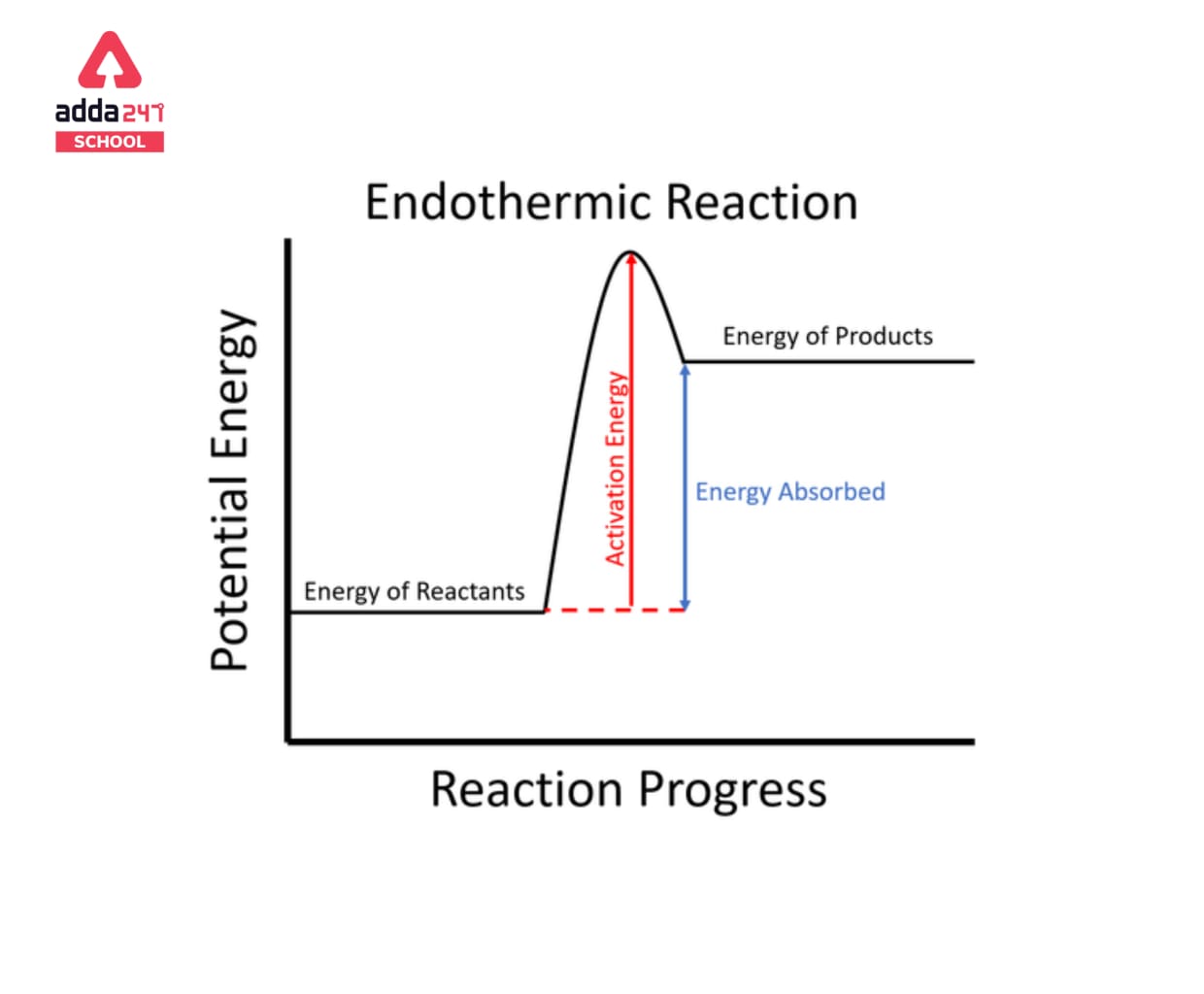
Endothermic processes require energy from the surrounding environment, preferably in the form of heat, in order to occur. Because endothermic processes absorb heat from their surroundings, they will produce a slight drop in temperature in their surroundings. They’re also non-spontaneous since endothermic reactions produce products that have more energy than the reactants. As a result, the enthalpy adjustment for an endothermic process is always positive. Heat is necessary for a chemical reaction involving the dissolution of ice, hence the process is endothermic. The concept is also commonly employed in physical disciplines, such as in chemical reactions, where warm energy (heat) is converted to chemical bond energy.
Exothermic Reaction Examples Equations
|
Reactants + Energy —> Products |
is the general equation for an endothermic reaction. Note that H stands for energy change. The temperature of the products in endothermic reactions is usually lower than the temperature of the reactants.
Melting ice cubes, solid salts, liquid water evaporation, and frost to water vapour conversion (melting, boiling, and evaporation are all endothermic processes).
10 Examples of Endothermic Reactions with Equations
Endothermic reactions are chemical reactions that absorb heat energy from their surroundings, resulting in a decrease in temperature. Here are 10 examples of endothermic reactions along with their balanced chemical equations:
- Melting of ice: H2O(s) → H2O(l) Heat is absorbed to convert ice into liquid water.
- Dissolving ammonium nitrate in water: NH4NO3(s) + H2O(l) → NH4NO3(aq) The dissolution process absorbs heat.
- Photosynthesis: 6 CO2(g) + 6 H2O(l) + light energy → C6H12O6(aq) + 6 O2(g) Photosynthesis in plants is an endothermic process because it requires energy from sunlight.
- Thermal decomposition of calcium carbonate: CaCO3(s) → CaO(s) + CO2(g) Heat is needed to break down calcium carbonate into calcium oxide and carbon dioxide.
- Ammonium chloride and water: NH4Cl(s) + H2O(l) → NH4Cl(aq) Dissolving ammonium chloride in water absorbs heat.
- Electrolysis of water: 2 H2O(l) → 2 H2(g) + O2(g) Electrolysis of water requires an input of electrical energy, making it endothermic.
- Reaction between barium hydroxide and ammonium thiocyanate: Ba(OH)2(aq) + 2 NH4SCN(aq) → Ba(SCN)2(aq) + 2 NH4OH(aq) Mixing barium hydroxide and ammonium thiocyanate results in an endothermic reaction.
- Sublimation of dry ice (solid carbon dioxide): CO2(s) → CO2(g) Dry ice sublimates at a temperature below room temperature, absorbing heat.
- Thermal decomposition of hydrogen peroxide: 2 H2O2(l) → 2 H2O(l) + O2(g) Breaking down hydrogen peroxide into water and oxygen requires heat.
- Reaction between ammonium nitrate and water: NH4NO3(s) + H2O(l) → NH4+ (aq) + NO3− (aq) Dissolving ammonium nitrate in water is an endothermic process.
These reactions all involve the absorption of energy in the form of heat or other forms (such as light or electricity) from their surroundings, resulting in a decrease in temperature.
Endothermic Reaction Process Formula for Class 10
In photosynthesis, plants are make the simple sugar glucose (C6H12O6) from carbon dioxide (CO2) and water (H2O). They also release oxygen (O2) in the process. The reactions of photosynthesis are summed up by.
6 CO2 + 6 H2O → C6H12O6 + 6 O2
Example of Endothermic Reaction Class 10
Endothermic reactions absorb heat from their surroundings, causing a decrease in temperature. Here are some examples:
-
Dissolution of Ammonium Nitrate: Absorbs heat, used in instant cold packs.
-
Photosynthesis: Plants absorb sunlight to convert CO2 and water into glucose.
-
Electrolysis of Water: Requires electricity to split water into hydrogen and oxygen.
-
Melting Ice: Absorbs heat to break hydrogen bonds in ice.
-
Evaporation: Liquids absorb heat when they change to gas, cooling the surroundings.
-
Baking Soda and Vinegar Reaction: Produces carbon dioxide and feels cold.
-
Ammunition Decomposition: Some explosives absorb heat during decomposition.
-
Reaction of Barium Hydroxide with Ammonium Chloride: Absorbs heat and lowers temperature.
-
Thermal Decomposition of Calcium Carbonate: Requires heat to break down into calcium oxide and carbon dioxide.
These are just a few examples of endothermic reactions, and there are many more in chemistry and biology. Endothermic reactions are characterized by a decrease in temperature and the absorption of energy from their surroundings.
Endothermic Reaction Examples in Real Life
- Sublimation of solid CO2
- Evaporation of liquid water, formation of water vapour.
- The baking of bread.
- The melting of ice to form water.
To keep cool, the human body uses the endothermic characteristic of evaporation. Sweating serves as a means of accomplishing this. Sweat (formed on the skin’s surface) absorbs heat from the skin and evaporates, resulting in a cooling effect.
Sweating, on the other hand, is not an exothermic response. Existing chemical bonds can be broken, new chemical bonds can be formed, or both can happen in a chemical reaction. Sweat evaporation does not result in any chemical changes, but it does result in a physical phase shift (from liquid to vapour). As a result, evaporation is classified as a physical rather than an endothermic reaction.
Examples of Endothermic Reactions vs. Exothermic Reactions
The phrases ‘Endo’ and ‘Exo,’ which imply ‘inside’ and ‘out,’ respectivelyGreek roots. The major difference between endothermic and exothermic reactions, as their names suggest, is that the former absorbs heat from the environment while the latter releases it.
An endothermic process is one that absorbs heat from its environment. As a result, all endothermic reactions and processes are endothermic. The opposite, however, is not true. Physical rather than chemical changes are involved in many endothermic reactions.
| S. No. | ENDOTHERMIC REACTION | EXOTHERMIC REACTION |
| 1. | The system absorbs heat from the surroundings | The system releases heat into the surroundings |
| 2. | The entropy of the surrounding decreases, i.e.
=> ΔS <0 |
Entropy of the surrounding increases, i.e.
=> ΔS>0 |
| 3. | Enthalpy change (ΔH) is positive | Enthalpy change (ΔH) is negative |
Related Post:
- Dielectric Constant- Definition, Formula, Meaning In Chemistry
- Limestone- Chemical Formula, Uses, Meaning
- Cannizzaro Reaction – Definition, Examples, Mechanism
- Lifecycle Of Silkworm- Diagram, Drawing, Project
- Scattering Of Light- Examples, Definition, Discovery, Prism
- Aufbau Principle Definition, Formula, Example, Limitation
- Area Of Sphere- Formula In Maths








 CUET PG MBA Question Paper 2026 PDF (Out...
CUET PG MBA Question Paper 2026 PDF (Out...
 NEET UG 2026 Registration Deadline, Syll...
NEET UG 2026 Registration Deadline, Syll...
 CBSE Class 10 Social Science Answer Key ...
CBSE Class 10 Social Science Answer Key ...














