Charle’s law: According to Charles’ law, the volume of an ideal gas is precisely proportional to the temperature in the absolute scale when the pressure is held constant. The law also specifies that the temperature measured in Kelvin and the volume of a dry gas sample will be in direct proportion to one another when the pressure placed on the sample remains the same. Jacques Charles, a French scientist, is credited with developing this principle in the year 1780. In his unpublished paper, he went into some detail describing this law.
Charle’s Law Definition
The Charles law is an experimental gas law that describes how gases have a tendency to expand when heated. This tendency is referred to as the law of volumes. The following is a contemporary expression of Charles’s law: If the pressure on a sample of a dry gas is maintained at a constant level, then the temperature in Kelvin and the volume will be in direct proportion to one another.
This indicates that the volume of a gas will also double if its temperature is increased by a factor of two. In the opposite direction, the volume of a gas will decrease by the same amount if its temperature is halved. The following is a mathematical expression that can be used to describe Charles’ law: V1/T1 equals V2/T2, where V1 and V2 are the starting and ending volumes of the gas, respectively. The initial temperature of the gas is denoted by T1, while the end temperature is denoted by T2. The Charles law is a particular instance of the ideal gas law, which is an equation that more generally represents the relationship between the pressure, volume, and temperature of any gas. The Charles law is a special case of the ideal gas law. The field of science and engineering both find several uses for Charles’s law. For instance, it is utilized in the development and calibration of thermometers, the forecasting of the behavior of gases in combustion engines, and the determination of the maximum volume of gas that can be contained in a particular container.
Charle’s Law Examples
Everyday examples of Charle’s law :
- Hot air balloons: Hot air balloons work by using the principle of Charles law. The air inside the balloon is heated, which causes it to expand. The expanded air has a lower density than the surrounding air, so the balloon rises.
- Helium balloons: Helium balloons also work by using Charles law. When a helium balloon is released, the helium inside the balloon expands as it warms up in the atmosphere. The expanded helium has a lower density than the surrounding air, so the balloon floats away.
- Tyres: The air inside tyres is also subject to Charles law. When tyres are inflated, the air inside them is heated by the friction of the tyres on the road. The expanded air has a higher pressure, which helps to keep the tyres inflated.
- Baking: When you bake bread or cake, the yeast inside the dough produces carbon dioxide gas. This gas causes the dough to rise, which is why bread and cake are so fluffy. The carbon dioxide gas expands as it is heated, which helps to make the baked goods rise even more.
- Soda cans: When you open a soda can, the carbon dioxide gas inside the can expands rapidly. This causes the soda to fizz and bubble. The carbon dioxide gas expands because it is being heated by the warm air outside of the can.
Charles Law Formula
The formula for Charle’s Law is as follows:
VI /TI = VF /TF
Where VI is the starting volume
VF = Final volume
TI = Initial absolute temperature
TF = Final absolute temperature
It is important to keep in mind that the temperatures listed below are absolute temperatures that are measured in Kelvin and not in degrees Fahrenheit or Celsius.
Charles Law Derivation
Since we are aware of the fact that, in accordance with Charle’s law, the volume of a fixed amount of dry gas is directly proportional to absolute temperature while the pressure is held constant, we may say that this statement is true. The assertion is capable of being expressed in the following manner:
V∝T
Because V and T are both independent variables, we may utilise the constant k to make them equivalent to one another.
V/T = constant = k
The value of k in this context is determined by the pressure of the gas, the amount of gas present, and the unit of volume used to measure the gas.
V*T = k——-(1)
Let us assume that the initial volume and temperature of a hypothetical perfect gas are denoted by V1 and T1, respectively.
The first equation can therefore be written down as
V1/T1=k——-(2)
As soon as that’s done, let’s adjust the temperature of the gas to T2. Alternately, in the event that its volume shifts to V2, then we can write,
V2/T2=k——–(3)
By combining the two equations up top, which are equations 2 and 3, we arrive at the desired result.
V1/T1=V2/T2
OR
V1T2=V2T1
It’s possible that you’re not aware of the fact that when you heat up a predetermined quantity of gas, that is, when you raise the temperature, the volume also rises. In a similar vein, bringing the temperature down brings about a reduction in the volume of the gas. In addition, the volume of the gas increases at a temperature of zero degrees centigrade by a factor of 1/273 of its initial volume for every degree that the temperature rises.For the sake of resolving issues that are associated with Charle’s law, it is essential to be aware of the fact that the unit of temperature must be in Kelvin, and not in Celsius or Fahrenheit as was previously said. The absolute temperature scale is also sometimes referred to as the temperature expressed in Kelvin. To convert the temperature from Celsius to Kelvin, simply add 273 to the temperature on the Celsius scale. This will give you the Kelvin temperature.
That must be in Kelvin given that Charles’ law, which stipulates that the volume (V) of the gas is precisely proportional to its temperature (T), states that the relationship between the two variables.
On the Kelvin scale, one degree Celsius of temperature change corresponds to one unit of temperature change on the Celsius scale. Always keep in mind that “Absolute Zero” corresponds to a value of -273 on the Kelvin scale.
When both the mass and the pressure remain the same, the temperature in Kelvin has an adverse effect on the density of the gas, which is proportional to the pressure.
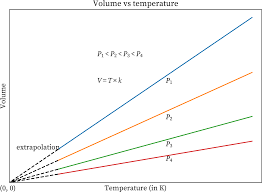
Charle’s Law Graph
The volume-temperature relationship is known as Charles’ law. According to this law, when the temperature of a gas is raised, the volume of the gas increases. As can be seen from the graph that has been shown below, the volume of the gas increases in proportion to the rise in temperature.
Charle’s Law Application
Here are some applications of Charle’s law.
1. Lungs of a human being are sponge-like organs that are filled with air and play an essential part in the process of respiration. When air enters the lungs, it causes the lungs to expand, and when the lungs contract, it causes air to flow out. During the winter, when the average temperature of the air is lower. As a direct consequence of this, the temperature of the body drops as well. According to Charles’ law, volume is exactly related to temperature. This law states that volume increases with increasing temperature. As a result, there is a correlation between the drop in temperature and the reduction in volume. On bitterly cold winter days, this causes the lungs to constrict, making it more difficult to engage in strenuous physical activities like jogging.
2. A pool float is something that every one of us used when we were younger and taking swimming lessons. Because they are filled with air rather than water, the pool floats have a density that is lower than that of water. When water is cooler than air, the temperature of the air inside pool floats drops, causing the air to contract. This causes the air inside the pool floats to shrink. This opposite phenomena takes place during the hot summer days when the water temperature is significantly higher.
3. Baking: The application of Charles’ law may also be seen in our own kitchens, especially when it comes to baking. Without the use of yeast, the delectable bakery goods that we offer, such as bread, cakes, and biscuits, would not be spongy and soft. Yeast is a type of leavening agent that works by converting the carbohydrates present in dough into carbon dioxide gas. The rate of conversion increases when baked goods like bread and cakes are produced. Because of the higher temperatures in the oven, the gas that is being released is expanding. Because of this expansion, the bread and cakes will have a spongy texture when they are baked.
Charle’s Law Exercise
Question 1: Find the initial volume of a gas at 800 K, if the ultimate volume is 4L at 200 K
Solution: According to the question, T1 = 800K
V2 = 4L
T2 = 200K
V1 =?
According to Charles law, V1/T1 = V2/T2 ———(1)
Subsisting to the value in the above equation, we get,
V1 / 8OO = 4 / 200
V1 = 16L
So the initial volume of the gas is 16L
Question 2:A sample of gas has an initial volume of 30.8 L and an initial temperature of -67 degree Celcius. What will be the temperature of the gas if the volume is 21.0 L?
According to the question,
V1 = 30.8 L
T1 =-67 degree celsius = 206 K
T2 = ?
V2 = 21.0L
According to Charle’s law,
V1/T1 = V2/T2
30.8/67 = 21/T2
T2 = 21*67/30.8
= 46.9⁰C
If V1 is the 3.60 L, T1 = 255 K, T2 = 102 K, then find the value of V2.
According to the question V2 =?
As we are aware of Charle’s law,
V1/T1 = V2/T2
3.60/255 = V2/102
V2 = 1.44L








 DTE Maharashtra Polytechnic Merit List 2...
DTE Maharashtra Polytechnic Merit List 2...
 How Normalisation Influence CUET Score 2...
How Normalisation Influence CUET Score 2...
 CUET Result 2025 OUT SHORTLY @cuet.nta.n...
CUET Result 2025 OUT SHORTLY @cuet.nta.n...









