The nitrogen cycle refers to the movement of nitrogen (N2) in different forms through the atmosphere. Nitrogen is a necessary component of all living things. All proteins and nucleic acids in human and animal worlds contain nitrogen atoms. Even though nitrogen gas constitutes 78 percent of the atmosphere by volume. This free nitrogen cannot be utilized by living things unless it is transformed into ammonia, nitrates, and other nitrogen-useful molecules. The nitrogen cycle is a cyclical process in which nitrogen moves from an inorganic form in the atmosphere to an organic form in living organisms. But nitrogen is eventually made accessible to plants, which in turn support all animal life, through a sequence of microbial changes. In this article, we will discuss the Nitrogen Cycle and different biochemical events in detail.
Nitrogen Cycle
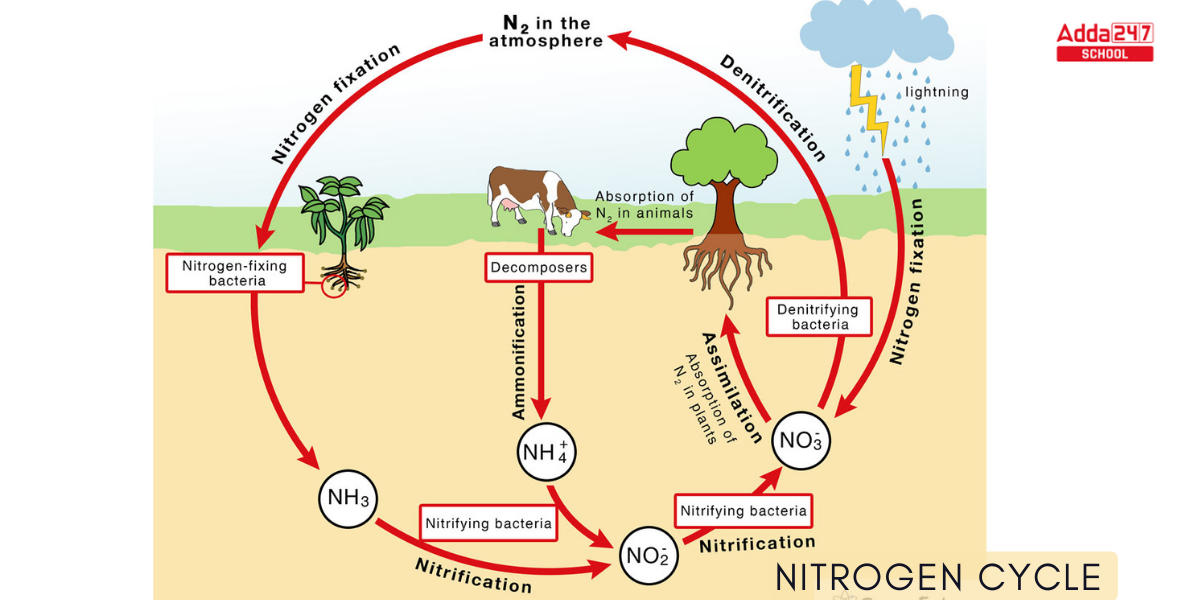
Nitrogen fixation, nitrogen assimilation, ammonification, nitrification, and denitrification are every step in the nitrogen cycle.
Nitrogen fixation, the conversion of nitrogen gas into inorganic nitrogen substances, is primarily (90%) performed by specific bacteria and blue-green algae. A far lesser amount of free nitrogen is fixed abiotically (through lightning, ultraviolet radiation, and electrical equipment) and in the Haber-Bosch process.
Microorganisms degrade the corpses of all living things—and their waste products—in the process of ammonification, which produces ammonia (NH3) and ammonium (NH4+).
Nitrification is the process by which nitrifying bacteria convert soil ammonia into nitrates (NO3) that plants can take into their own tissues.
Denitrifying bacteria, which are particularly active in damp anaerobic soils, also metabolize nitrates. These bacteria reduce soil nitrates by generating free atmospheric nitrogen and this is called denitrification of Nitrogen.
Nitrogen Cycle Class 8
The nitrogen cycle is an important natural process that explains how nitrogen moves between the atmosphere, soil, plants, animals, and microorganisms. Here’s a simplified explanation suitable for Class 8:
Steps of the Nitrogen Cycle:
Nitrogen Fixation:
Nitrogen gas (N₂) makes up about 78% of Earth’s atmosphere, but plants and animals can’t use nitrogen in its gaseous form. Nitrogen fixation is the process that converts nitrogen gas into forms that living organisms can absorb and use.
This is done mainly by bacteria found in the soil or in the roots of certain plants (like legumes). These bacteria convert nitrogen gas into ammonia (NH₃), which plants can absorb.
Nitrification:
The ammonia (NH₃) produced during nitrogen fixation is then converted into nitrites (NO₂⁻) and nitrates (NO₃⁻) by bacteria in the soil. These nitrates are what plants use to make proteins and other essential compounds.
Assimilation:
Plants absorb the nitrates from the soil through their roots. They use these nitrogen compounds to make proteins and other compounds, which are passed on to animals when they eat the plants.
Ammonification (Decomposition):
When plants, animals, and other organisms die, decomposers (like bacteria and fungi) break down their bodies. This process releases nitrogen back into the soil in the form of ammonia.
Denitrification:
Some soil bacteria convert nitrates back into nitrogen gas (N₂), which is released into the atmosphere. This completes the nitrogen cycle, allowing nitrogen to be reused.
Importance of the Nitrogen Cycle:
Nitrogen is an essential element for all living things as it is a key component of DNA, proteins, and chlorophyll.
The nitrogen cycle ensures that nitrogen is constantly recycled and made available to living organisms.
This cycle is crucial for maintaining the balance of nitrogen in the environment.
Nitrogen Cycle Diagram
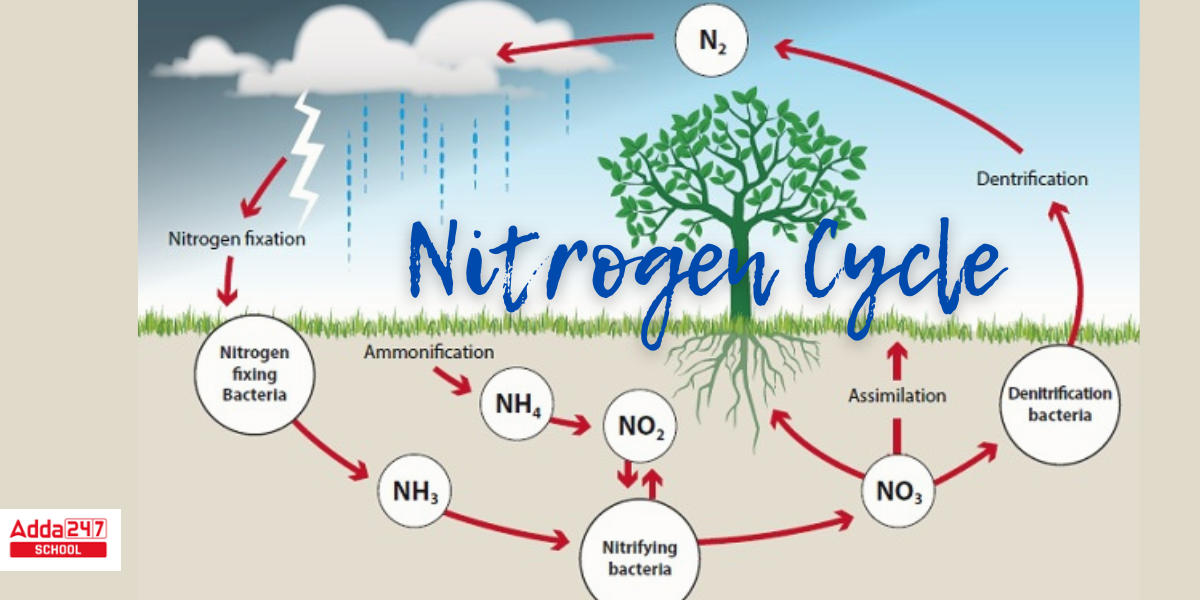
Nitrogen is required by many biomolecules, including DNA, protein, and chlorophyll. In the ecosystem, nitrogen undergoes numerous transformations, moving from one form to a different one as organisms in the form of nitrogen cycle use it for development and, in some circumstances, energy. Nitrogen fixation, nitrification, denitrification, nitrogen assimilation, and ammonification are the principal nitrogen reactions. The transition of nitrogen into a multitude of oxidation states is critical to biosphere production and is heavily reliant on the activities of a wide array of microorganisms such as bacteria, archaea, and fungi. A Nitrogen Cycle Diagram with detailing for better understanding is given below.
Nitrogen Fixation Drawing
Nitrogen fixation Drawing is given below.
Nitrogen Fixation
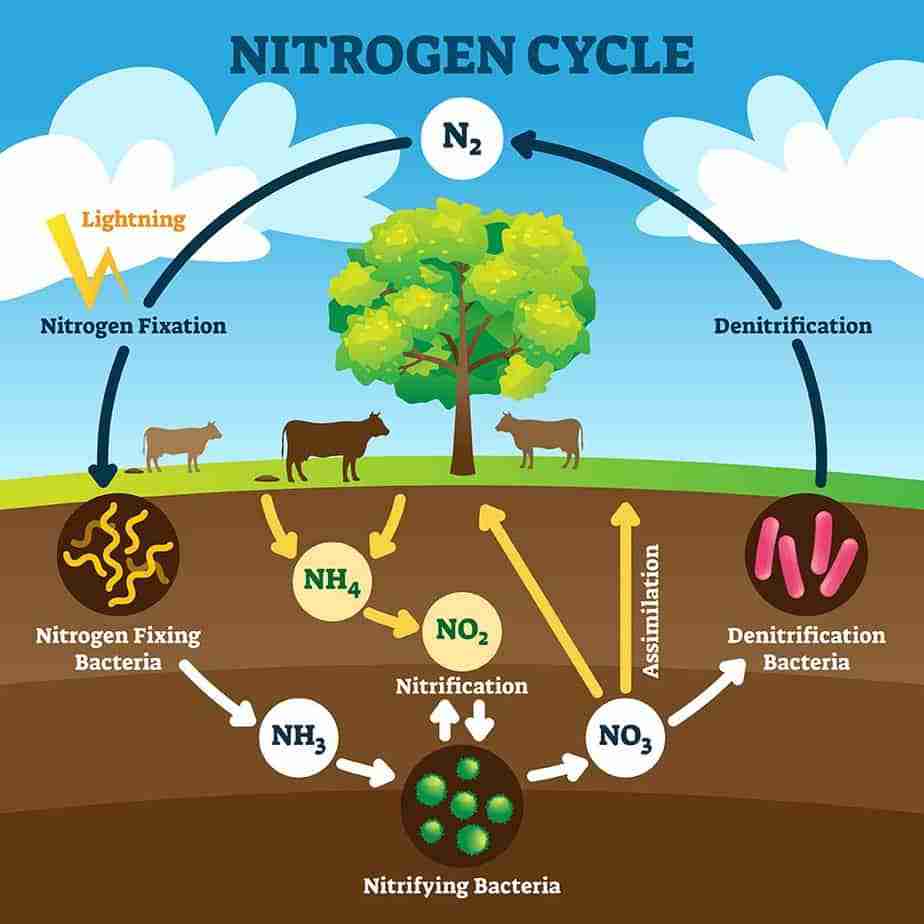
Nitrogen Fixation Cycle
The nitrogen cycle is a biogeochemical process through which nitrogen is changed into a variety of forms before returning to the atmosphere after first entering the soil and then organisms. It involves a number of chemical reactions, including the fixation of nitrogen, nitrification, denitrification, decay, and putrefaction. Both organic and inorganic forms of nitrogen gas are known to exist. Living things contain organic nitrogen, which is transferred along the food chain when they consume other living things.
In the atmosphere, inorganic forms of nitrogen are prevalent. Symbiotic bacteria that can transform the inert nitrogen into useful forms, such as nitrites and nitrates, make this nitrogen available to plants.
What is a Stethoscope?- Its Parts, Uses, Diagram
Stages of Nitrogen Fixation Cycle
Nitrogen fixation, nitrification, assimilation, ammonification, and denitrification are the steps that make up the nitrogen cycle.
- The nitrogen cycle’s first stage is represented by it. Here, atmospheric nitrogen (N2) is transformed into ammonia, an useful molecule that is predominantly available in an inert form (NH3).
- The inert form of nitrogen gas is deposited into soils during the nitrogen fixation process from the atmosphere and surface waters, primarily through precipitation.
- Symbiotic bacteria known as diazotrophs finish the full nitrogen fixation process. A significant part of this process is also played by Azotobacter and Rhizobium. The nitrogenase enzyme found in these bacteria can combine gaseous nitrogen and hydrogen to produce ammonia.
- Either atmospheric fixation, which includes lightning, or industrial fixation, which involves producing ammonia under extreme heat and pressure, are two ways that nitrogen can be fixed. This can also be repaired by using artificial processes, especially industrial ones that produce ammonia and fertilizers high in nitrogen.
Nitrogen Fixation Example
- In nature, nitric oxide is fixed, or mixed, as nitric oxide by lightning and ultraviolet radiation, but larger quantities of nitrogen are fixed by soil microorganisms as ammonia, nitrites, and nitrates.
- They influence more than 90% of all nitrogen fixing. The cyanobacteria (or blue-green algae) Anabaena and Nostoc, as well as genera like Azotobacter, Beijerinckia, and Clostridium, are examples of free-living (nonsymbiotic) bacteria.
- Mutualistic (symbiotic) bacteria include Rhizobium, which is linked to leguminous plants, and various Azospirillum species, which are linked to cereal grasses.
- The nitrogen-fixing bacteria that live in symbiosis infiltrate the host plants’ root hairs, where they multiply and encourage the growth of root nodules, which are enlargements of plant cells and bacteria that are closely bonded.
- The bacteria in the nodules convert free nitrogen to ammonia, which the host plant uses for growth.
- Seeds are typically inoculated with commercial cultures of suitable Rhizobium species, especially in soils deficient in or lacking the necessary bacterium, to ensure sufficient nodule formation and optimum growth of legumes (such as alfalfa, beans, clovers, peas, and soybeans).
Nitrogen Fixation in Nitrogen Cycle
Nitrogen fixation is the first phase in the nitrogen cycle. Atmospheric nitrogen (N2), which is mostly inactive, is transformed into the useful form -of ammonia (NH3). Nitrogen in the air exists in the stable form, N2. It needs to be fixed by bacteria, lightning in the sky that fix nitrogen before an organism may absorb it.
- Nitrogen Fixation by Bacteria – Nitrogen-fixing bacteria are mostly symbiotic bacteria known as Diazotrophs and blue-green algae that fix nitrogen gas from the atmosphere into organic nitrogen. They transform it into nitrogen molecules that are then released into the soil. Nitrogen-fixing bacteria can be found in soil and leguminous plant root nodules. Precipitation introduces nitrogen into the soil and surface waterways. When it settles in water or soil, it undergoes changes that cause the link between N2 to be broken.
- As a consequence, the free electrons in N2 are bound by hydrogen from the water to create NH2+. Ammonium is the product of this reaction. The microorganisms found in the soil complete this process. Many microorganisms, primarily Rhizobium bacteria, complete this process. The chemical mechanism that occurs is — 3[CH2O] + 2N2 + 3H2O + 4H+ 3CO2 + 4NH4+ 3CO2 + 4NH4+
- Atmospheric nitrogen fixation – Aside from the nitrogen-fixing bacteria, A natural occurrence in which lightning energy splits nitrogen into nitrogen oxides, which are then utilized by plants.
- Industrial Nitrogen Fixation – It is a substitute created by humans in which atmospheric nitrogen is transformed into ammonia by Haber’s process and then into nitrates in different fertilizers. The immediate interaction between nitrogen and hydrogen produces ammonia. It is then turned into various fertilisers, including urea.
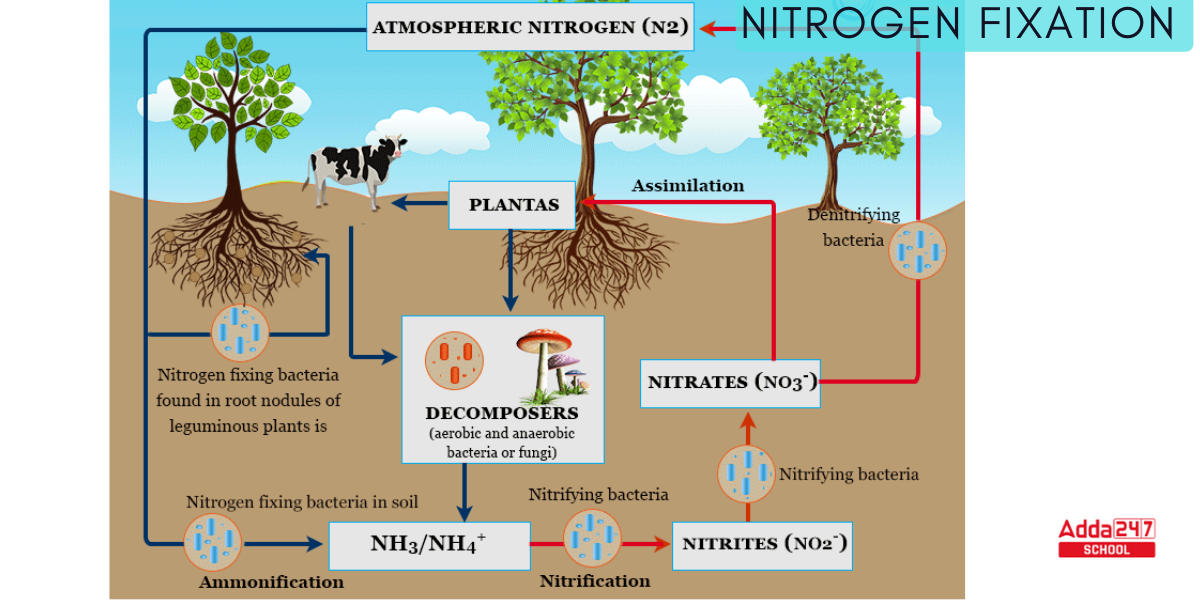
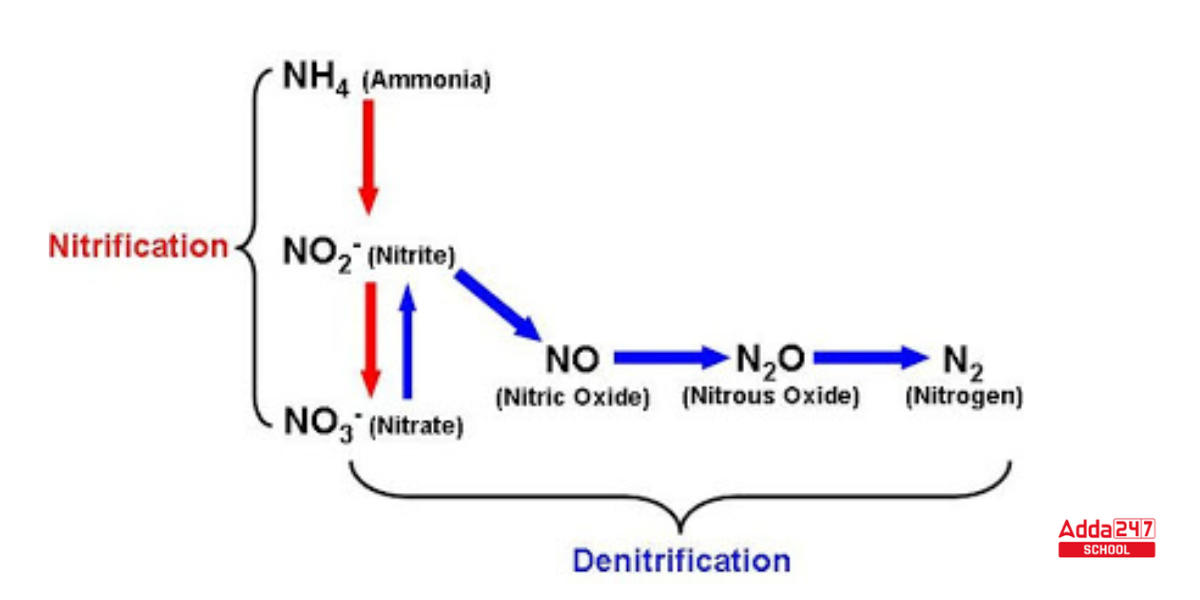
Nitrification
Nitrosomonas and Nitrococcus bacteria convert ammonia to nitrite during this Nitrification process (Second Step of the Nitrogen cycle). Nitrites are generated through the oxidation of ammonia by Nitrosomonas bacterium species. All of these bacteria, known as nitrifying bacteria, live in the soil.
- The process provides energy to the bacteria by turning nitrogen into a useful form. They require oxygen to complete the procedure.
- Nitrobacter transforms the nitrites generated into nitrates. This conversion is critical since ammonia gas is poisonous to plants. The following is the reaction implicated in the Nitrification process:
2NH3 + 3O2 → 2NO2– + 2H+ + 2H2O (nitrite )
2NO2– + O2 → 2NO3– (nitrate)
Assimilation Nitrogen Cycle
- The generated nitrates in the early stage of the nitrogen cycle (in soil) are taken by the plants via their root system during the Assimilation process. These chemicals are converted into protein from plants and other organic components that make up their body.
- Nitrates are found in plants and are absorbed by consumers before passing through the alimentary chain and entering the food web. Nitrate and other nitrogen molecules are absorbed through assimilation. The NH3, NO3-, and NO3- are incorporated into plant and animal biological tissues and used in cellular functions. These nitrogen compounds are required for the production of important biomolecules.
Ammonification
- Ammonification is the process by which organic nitrogen is converted into ammonium with the help of bacteria and fungi. The ammonium generated can be utilized in other plant biological processes. When plants or animals die, the nitrogen in the organic matter returns to the soil. Complex nitrogen molecules are also converted into simple organic molecules via animal feces.
- Organic matter is converted back into ammonium by decomposers, which are bacteria or fungi found in the soil. This breakdown process generates ammonia, which is then utilized in other biological activities.
Denitrification Nitrogen Cycle
- Some nitrogen molecules are decomposed during the denitrification process in the Nitrogen cycle. This is the final phase in which the nitrogen compounds existing in the soil are returned to the atmosphere. Some nitrates are not absorbed by plants. Pseudomonas and Clostridium assist in turning them into atmospheric nitrogen.
- Denitrification is the process of converting NO3 and NO2 into N2 and then recycling that N2 into the atmosphere. This is also known as nitrogen fixation in reverse. The anaerobic process happens in regions where there is no oxygen, such as deep soils and deep seas. The chemical reaction that occurs in this stage is —- 4NO3– + 5[CH2O] + 4H+→ 2N2↑ + 5CO2 ↑ + 7H2O
Nitrogen Cycle in Nature and Ocean Important Facts
Here we have discussed some Importance of the nitrogen cycle as follows.
- The nitrogen cycle aids plants in the production of chlorophyll from nitrogen molecules. It contributes to the plants’ stable and optimal growth.
- Through the metabolic process, the nitrogen cycle aids in the conversion of inert nitrogen gas into a useful form for plants.
- Bacteria participate in the decomposition of animal and plant materials during the ammonification process, which helps to clean up the environment.
- nitrogen cycle aids in the manufacture of natural and synthetic fertilizers, such as ammonium sulfate.
- Nitrogen is an essential ingredient for all living things. It is responsible for the formation of proteins and nucleic acid.
- Life could not survive without nitrogen molecules. The nitrogen cycle keeps the amount of nitrogen in the atmosphere stable.
- When nitrogen from fertilizers in the soil is washed away, the process of depositing nitrogen in water bodies is known as eutrophication.
- The soil is enriched with the essential nutrients needed for cultivation as a result of the release of nitrates and nitrites.








 Omnivores Animals- Definition, Name List...
Omnivores Animals- Definition, Name List...
 Nephron: Definition, Diagram, Structure,...
Nephron: Definition, Diagram, Structure,...
 Life Processes: Check Nutrition, Transpo...
Life Processes: Check Nutrition, Transpo...









