The Adda247 provides you with the Class 12 Chemistry NCERT solutions. Chapter 5 of the NCERT class 12 chemistry is Coordination Compounds. The compounds dealt with here, are relatively and the concepts are novel, and therefore the students are required to pay special attention to the chapter.
The chapter explains Werner’s theory of Coordination compounds, Bonding in Coordination compounds, Nomenclature, Isomerism and others. The chapter can be well understood through the exercises and solutions, that help to reinforce the fundamentals.
Class 12 Chemistry Chapter 5 NCERT Solutions
The process of organizing people or groups so that they work together properly and well.
Coordination Compounds include such substances as vitamin B12, hemoglobin and chlorophyll, dyes and pigments, and catalysts used in preparing organic substances.
Coordination compounds contain a central metal atom surrounded by nonmetal atoms or groups of atoms, called ligands.
Importance of Coordination Compounds;
Coordination Compounds are a major feature of the chemistry of over half the elements.
Coordination compounds have important roles as industrial catalyst in controlling reactivity, and they are essential in biochemical processes.
Coordination compounds generally display a variety of distinctive physical and chemical properties, such as color, magnetic susceptibility, solubility and volatility, and ability to undergo oxidation-reduction reactions, and catalytic activity.
NCERT Solutions for Class 12 Chemistry Chapter 5 help students learn about “coordination compounds” and understand the concepts related to them. However, this chapter which is included in the class 12 chemistry syllabus is an important concept as we talk about atoms and chemical reactions. Chapter 5 explains different compounds formed from transition metals.
The unique properties of these compounds are discussed in this chapter. Some of the topics that you will study here are the naming of coordination compounds in the IUPAC system and finding the corresponding chemical formulae when an IUPAC name is given. Chapter 5 also discusses essential terms like ligands, central atom, and coordination entities along with coordination number.
NCERT Solutions Class 12 Chemistry Chapter 5 Coordination Compounds
Q) Explain the bonding in coordination compounds in terms of Werner’s postulates.
Answer: Following are the postulates of Werner’s theory:
- In coordination compound metals shows two types of linkages – primary and secondary.
- The primary valances are normally ionisable and are satisfied by negative ions.
- The secondary valances are non-ionisable. These are satisfied by neutral molecules or negative ions. The secondary valances is equal to the coordination no. and is fixed for a metal.
- The ions/groups bound by the secondary linkages to the metal have characteristics spatial arrangements corresponding to different coordination numbers.
Q) FeSO4 solution mixed with (NH4)2SO4 solution in 1 : 1 molar ratio gives the test of Fe2+ ion but CuSO4 solution mixed with aqueous ammonia in 1 : 4 molar ratio does not give the test of Cu2+ ion. Explain why?
Answer: FeSO4 does not form any complex with (NH4(SO4). Instead, they form a double salt, FeSO4.(NH4)2SO4.6H2O (Mohr salt) which dissociates completed in ion in the solution. Hence, it gives the test of Fe2+ ions. CuSO4 solution mixed with aqueous ammonia in 1 : 4 molar ratio forms the complex with the formula [Cu(NH3)]SO4 in which the complex ion, [Cu(NH3)4]2+ does not dissociate to give Cu2+ ions. Hence, it does not give the tests of Cu2+ ion.
Q) Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Answer:
Coordination entity: Coordination entity constitutes a central metal atom or ion bounded to a fixed no. of ions or molecules.
For example, [CoCl3(NH3)3], [Ni(CO)4].
Ligand: The ions or molecules bound to the central atom/ion in the coordination entity are called ligands.
For example, H2o, NH3.
Coordination number: The coordination number of a metal ion in complex can be defined as the no. of ligand donor atom to which the metal is directly bonded.
For example, In PtCl6]2-, the coordination no. of Pt is 6. In [Ni(NH3)4]2+, the coordination no. of Ni is Coordination polyhedron: The spatial arrangement of the ligand atoms which are directly attached to the central atom/ion defines a coordination polyhedron about the central atom.
For example, [PtCl4]2- is square planar, [Ni(CO)4] is tetrahedral.
Homoleptic complexes: Complexes in which a metal is bound to only one kind of donor group are known as homoleptic complexes.
For example, [Co(NH3)6]3+
Heteroleptic complexes: Compleses in which a metal is bound to more than one kind of donor groups are known as heteroleptic complexes.
For example, [Co(NH3)4Cl2]+.
Q) What is meant by unidentate, didentate and ambidentate ligands? Give two examples of each.
Answer:
Unidentate ligands: When a ligand is bound to a metal ion through a single donor atom, the ligand is known as unidentate ligand.
For example, Cl–, H2O.
Didentate ligand: When a ligand can be bind through two donor atoms, the ligand is said to be didentate ligand.
For example, (ethane-1,2-diamine), (oxalate).
Ambidentate ligand: Ligand which can ligate through two different atoms is called ambidentate ligand.
For example, NO2–, SCN–.
Q) Specify the oxidation numbers of the metals in the following coordination entities:
(i) [Co(H2O)(CN)(en)2]2+
(ii) [CoBr2(en)2]+
(iii) [PtCl4]2-
(iv) K3[Fe(CN)6]
(v) [Cr(NH3)3Cl3]
Answer: Let x be the oxidation no. of metal in the following compounds;
(i) X + 0 + (-1) + 2(0) = +2
X – 1 = 2
X = 1
(ii) X + 2(-1) +2(0) = +1
X – 2 = 1
X = 3
(iii) X + 4(-1) = -2
X – 4 = -2
X = +2
(iv) 3(+1) + x + 6(-1) = 0
3 + x + (-6) = 0
X = 3
(v) X + 3(0) + 3(-1) = 0
X + (-3) = 0
X = 3
Q) Using IUPAC norms write the formula of following:
- Tetrahydroxozincate(II)
- Potassium tetrachloridopalladate(II)
- Pottasium tetracynanickelate(II)
- Diamminedichloridoplatinum(II)
- PENTAamminenitrito- O- cobalt(III)
- Hexaamminecobalt(III) sulphate
- Potassium tri(oxalate)chromate(III)
- Hexaammineplatinum(IV)
- Tetrabromidocuprate(II)
- Pentaamminenitrito-N-cobalt(III)
Answer:
- [Zn(OH)4]2-
- K2[PdCl4]
- [Pt(NH3)2Cl2]
- K2[Ni(CN)4]
- [Co(ONO)(NH3)5]2+
- [Co(NH3)6]2(SO4)3
- K3[Cr(C2O4)3]
- [Pt(NH3)6]4+
- [Cu(Br)4]2-
- [Co(NO2)(NH3)5]2+
Q) Using IUPAC norms write the systematic names of the following:
- [Co(NH3)6]Cl3
- [Pt(NH3)2Cl(NH2CH3)]Cl
- [Ti(H2O)6]3+
- [CO(NH3)4Cl(NO2)]Cl
- [Mn(H2O)6]2+
- [NiCl4]2-
- [Ni(NH3)6]Cl2
- [CO(en)3]3+
- [Ni(CO)4]
Answer:
- Hexaamminecobalt(III) chloride
- Diamminechlorido(methylamine) platinum(II) chloride
- Hexaquatitanium(III) ion
- Tetraamminichloridonitrito-N-Cobalt(III) chloride
- Hexaquamanganese(II) ion
- Tetrachloridonickelate(II) ion
- Hexaamminenickel(II) chloride
- Tris(ethane-1, 2-diammine) cobalt(III) ion
- Tetracarbonylnickel(0)
Q) Give the oxidation state, d orbital occupation and coordination no. of the central metal ion in the following complexes:
- K3[Co(C2O4)3]
- (NH4)2[CoF4]
- cis-[Cr(en)2Cl2]Cl
- [Mn(H2O)6]SO4
Answer:
- K3[Co(C2O4)3]
The central metal ion is Co
Its coordination no. is 6
The oxidation state of Co is
X – 6 = -3x = +3
The d orbital occupation for Co3+ is t2g6eg0
- (NH4)2[CoF4]
The central metal ion is Co
The coordination no. is 4
The oxidation state of Co is
X – 4 = -2
X = +2
The d orbital occupation for Co2+ is eg4t2g3
- cis-[Cr(en)2Cl2]Cl
the central metal ion is Cr
its coordination no. is 6
The oxidation state of Cr
X + 2(0) + 2(-1) = 1
X = +3
The d orbital occupation for Cr3+ is t2g3
- [Mn(H2O)6]SO4
The central metal ion is Mn
Its coordination no is 6
The oxidation state of Mn
X + 2 = 0
X = 2
The d orbital occupation for Mn2+ is t2g3eg2
Q) Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also give stereochemistry and magnetic moment of the complex:
- K[Cr(H2O)2(C2O4)2].3H2O
- [Co(NH3)5Cl]Cl2
- CrCl3(py)3
- Cs[FeCl4]
- K4[Mn(CN)6]
Answer:
| Compound | IUPAC name | Oxidation state | Electronic configuration | Coordination number | Stereochemistry | Magnetic moment |
| K[Cr(H2O)2(C2O4)2].3H2O
|
Potassium diaquabisoxalatochromate (III)trihydrate | +3 | [Ar]3d3 | 6 | Cis and trans | 3.87 |
| [Co(NH3)5Cl]Cl2
|
Pentaminechorocobaltate(III)chloride | +3 | [Ar]3d6 | 6 | No stereoisomerism | 4.90 |
| CrCl3(py)3
|
Trichlorotripyridine chromate(III) | +3 | [Ar]3d3 | 6 | Fac. And mar. isomers | 3.87 |
| Cs[FeCl4]
|
Caseium tetrachloroferrate(III) | +3 | [Ar]3d5 | 4 | No | 5.92 |
| K4[Mn(CN)6]
|
Potassium hexacynomanganate(II) | +2 | [Ar]3d5 | 6 | no | 5.92 |
Q) What is meant by stability of a coordination compound in solution? State the factors which govern stability of complexes.
Answer: The stability of coordination compounds in solution means the degree of association between the metal ion and the ligands involved in the state of equilibrium. Quantitatively, the stability is expressed by the equilibrium constant for the association.
M +3L -à ML3
Stability Constant, = [ML]/[M][L3]
For this reaction., the greater the value of the stability constant, the greater is the proportion of ML3 in the solution.
Q) Write the formulas for the following coordination compounds:
(i)Tetraamminediaquacobalt(IlI) chloride
(ii)Potassium tetracyanidonickelate(II)
(iii)Tris(ethanp-1,2-diamine) chromium(III) chloride
(iv)Amminebromidochloridonitrito-N- platinatc(II)
(v)Dichloridobis(ethane-l ,2-diamine) platinum (IV) nitrate
(vi)Iron(III)hexacyanidoferrate(II)
Ans: (i) [CO(NH3)4(H2O)2]Cl3.
(ii)K2[Ni(CN)4]
(iii)[Cr(en)3]Cl3
(iv)[Pt (NH3) Br Cl (N02)]–
(v)[PtCl2(en)2](N03)2
(vi)Fe4[Fe(CN)6]3
Q) Give evidence that [Co(NH3)5Cl]S04 and [Co(NH3)5S04]Cl are ionisation isomers.
Ans: When dissolved in water, they give different ions in solution which can be tested by adding AgN03 solution and BaCl2 solution, i.e.,
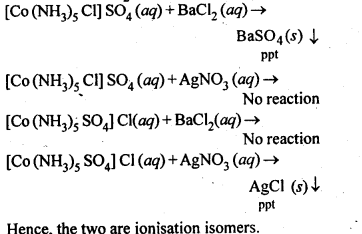
Q) Write IUPAC names of following co-ordination compounds :
(a) [CO(NH3)6]Cl3
(b) [CO(NH3)Cl]Cl2
(C) K3[Fe(CN)6]
(d) [K3[Fe(C2O4)3]
(e) K2[PdCl4]
(f) [Pt(NH3)2ClNH2CH3]Cl
Ans: (a) hexaamminecobalt (III) chloride
(b) pentaamminechloridocobalt (III) chloride
(c) potassium hexacyanoferrate (III)
(d) potassium trioxalatoferrate (III)
(e) potassium tetrachloridoplatinum (II)
(f) diamminechlorido (methylamine) platinum(II) chloride.
| Related Chapters |










 CUET UG Final Answer Key 2025 Revised, D...
CUET UG Final Answer Key 2025 Revised, D...
 DU Cut off 2025, Delhi University Expect...
DU Cut off 2025, Delhi University Expect...
 OUAT Result 2025 OUT @ouat.nic.in: Check...
OUAT Result 2025 OUT @ouat.nic.in: Check...

