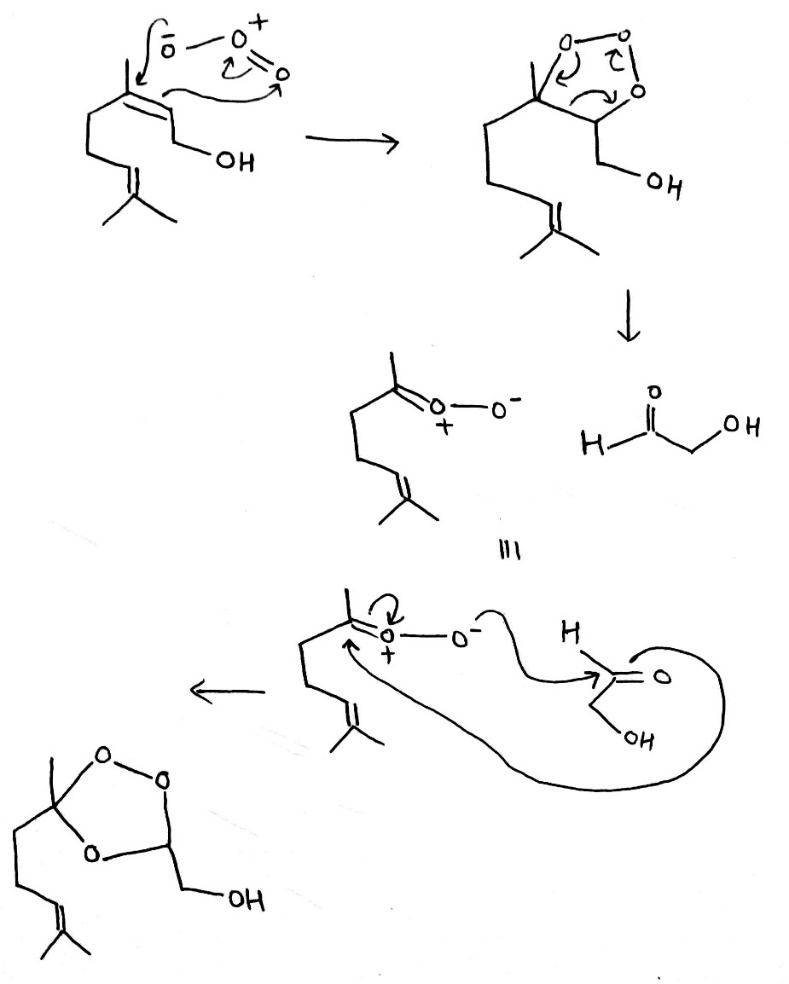
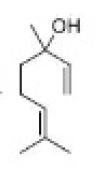
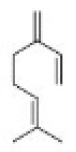
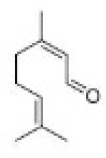
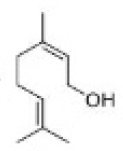
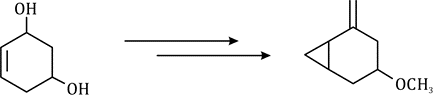
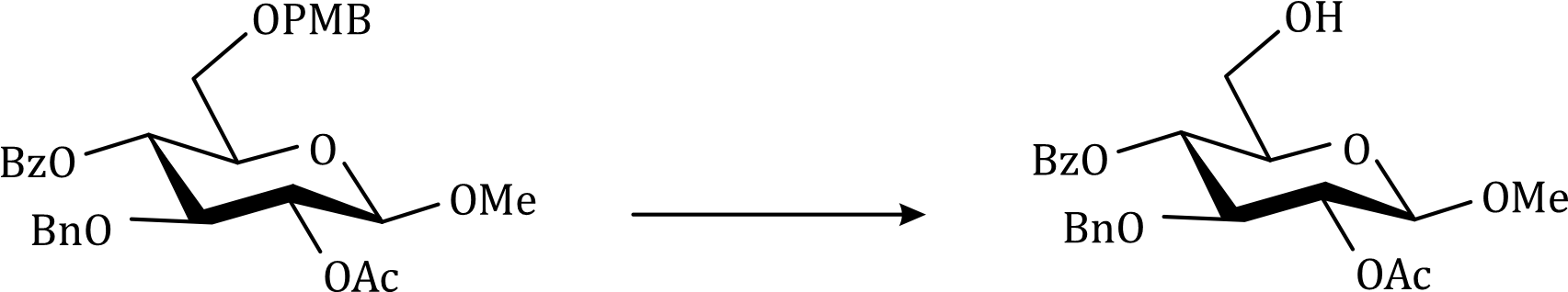
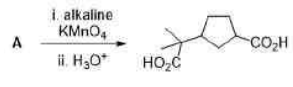
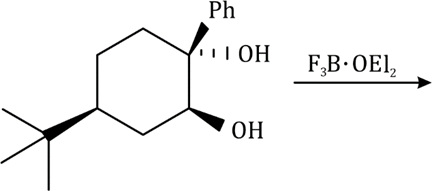
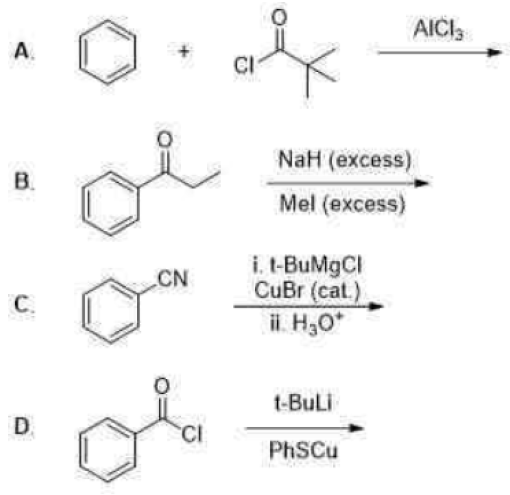
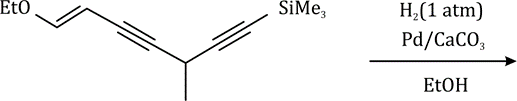Correct option is D
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds are cleaved with ozone (O3 ).Multiple carbon–carbon bond are replaced by carbonyl (C=O) groups, such as aldehydes, ketones, and carboxylic acids. The reaction is predominantly applied to alkenes, but alkynes and azo compounds are also susceptible to cleavage. The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions.
Alkenes can be oxidized with ozone to form alcohols, aldehydes or ketones, or carboxylic acids. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol at −78 °C until the solution takes on a characteristic blue color, which is due to unreacted ozone. Industry however recommends temperatures near −20 °C. This color change indicates complete consumption of the alkene.