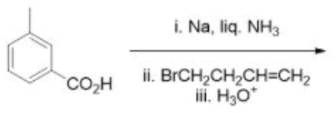
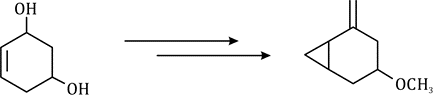
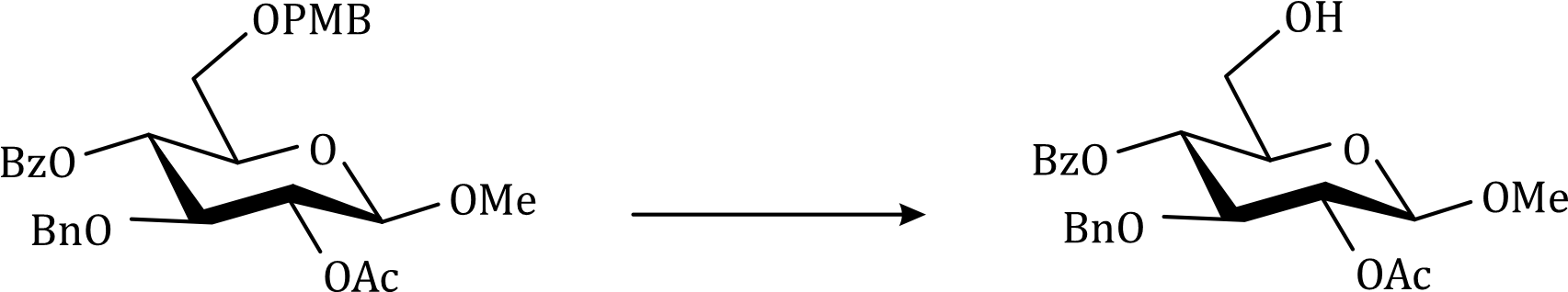
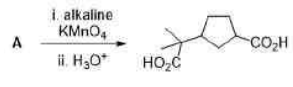
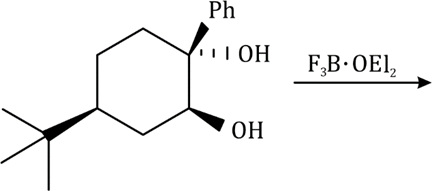
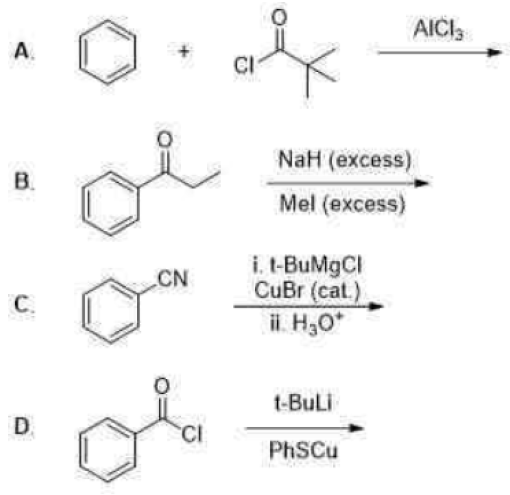
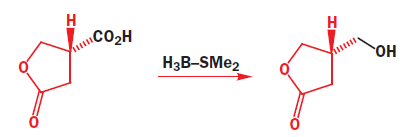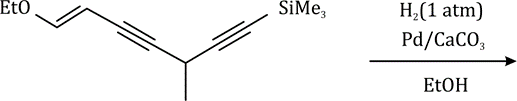Correct option is B
Selectivity comes in three sorts: chemoselectivity, regioselectivity, and stereoselectivity. Chemoselectivity is which group reacts; regioselectivity is where it reacts. Stereoselectivity is how the group reacts with regard to the stereochemistry of the product.
Sodium ethoxide, also referred to as sodium ethanolate, is the ionic, organic compound with the formula CH3CH2ONa, C2H5ONa, or NaOEt (Et = ethyl). Sodium ethoxide is commonly used as a base in the Claisen condensation and malonic ester synthesis. The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base. The reaction produces a β-keto ester or a β-diketone. The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the alkoxide is regenerated.
The way to get regioselective addition directly to the carbonyl group is to add a hard, Lewis-acidic metal salt, such as CeCl3.
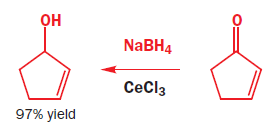
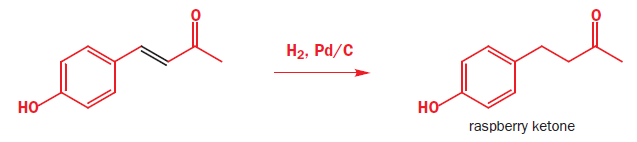
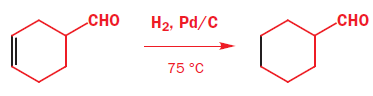
It should not surprise you that regioselective reduction of the C=C double bond alone is best done using catalytic hydrogenation as the C=C bond is weaker than the C=O bond. The flavouring compound known as ‘raspberry ketone’ is made by this method.