Table of Contents
Markovnikov rule: The outcome of various chemical addition processes can be described using Markovnikov’s Rule, also known as Markownikoff’s rule. In 1865, the Russian scientist Vladimir Vasilyevich Markovnikov proposed this rule.
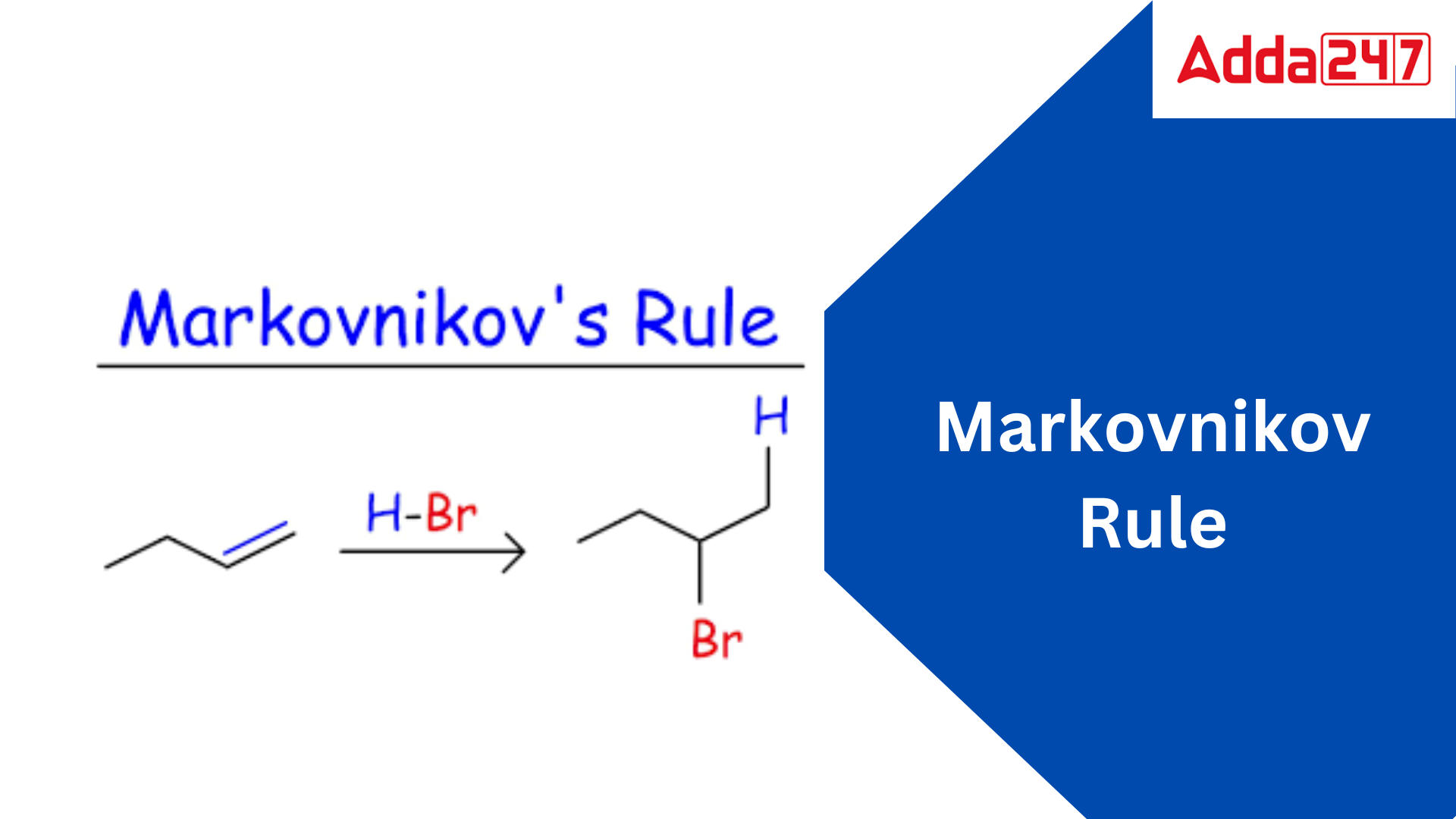
Markovnikov Rule
Markovnikov’s rule is an organic chemistry rule of thumb that predicts the regiochemistry of addition reactions to alkenes. The rule states that when a protic acid HX or other polar reagent is added to an asymmetric alkene, the hydrogen atom (H) or electropositive part bonds to the carbon atom with fewer hydrogen atoms bonded to it, while the halide (X) group bonds to the carbon atom with more hydrogen atoms bonded to it.
For example, adding HBr to propene (CH3CH=CH2) yields 2-bromopropane (CH3CHBrCH3) rather than 1-bromopropane (CH3CH2CH2Br). This is due to the fact that the hydrogen atom in HBr is more electropositive than the bromine atom, and so it is more likely to attach to the carbon atom with less hydrogen atoms linked to it. Because the bromine atom is more electronegative than the hydrogen atom, it is more likely to connect to the carbon atom when there are more hydrogen atoms attached to it. The image is given below for illustration.
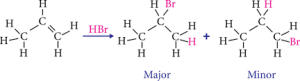
Markovnikov’s rule is not always followed, as a variety of circumstances might impact an addition reaction’s regiochemistry. It is, nonetheless, a useful rule of thumb for predicting the results of addition reactions to alkenes.
What is the Mechanism Behind Markovnikov’s Rule?
Markovnikov’s rule’s mechanism is based on the concept of carbocation stability. A carbocation is a positively charged, electron-deficient carbon atom. A carbocation is more stable the more replaced it is. The protonation of the alkene is the initial step in the addition of a protic acid HX to an alkene. This results in the formation of a carbocation, which is then attacked by the halide ion X. Because the halide ion is more electronegative than the hydrogen atom, it is more likely to bond to the carbon atom in the presence of additional hydrogen atoms. This is due to the fact that the carbon atom with more hydrogen atoms is less electron deficient and so less likely to take the electron from the halide ion.
Markovnikov’s rule mechanism can be summarised as follows:
- The protic acid HX protonates the alkene, resulting in the formation of carbonation.
- The product is formed when the halide ion X hits the carbocation.
The relative stability of the two carbocations that can be generated determines the result of the addition reaction. Because the carbocation with the most substituents is the most stable, the halide ion is more likely to attach to the carbon atom with the most hydrogen atoms bound to it.
Here’s an illustration of how Markovnikov’s rule works:
When HBr is added to propene (CH3CH=CH2), 2-bromopropane (CH3CHBrCH3) is formed. The alkene is protonated by HBr to generate a carbocation in the first step. The bromide ion then attacks the carbocation, producing 2-bromopropane.The more stable carbocation is the one with the most substituents. The carbocation with the most substituents in this example is the one with two hydrogen atoms bound to it. As a result, the bromide ion is more likely to connect to the carbon atom with one hydrogen atom attached, producing 2-bromopropane.
Anti Marknovnikov Rule Addition Reactions
Anti-Markovnikov addition reactions are a type of addition reaction in which the hydrogen atom (H) or electropositive part bonds to the carbon atom, while the halide (X) group or electronegative part bonds to the carbon atom with fewer hydrogen atoms bonded to it. This is the inverse of what Markovnikov’s rule predicts.
Free radicals generally catalyse anti-Markovnikov addition reactions. Free radicals are chemical entities with an unpaired electrons that are extremely reactive. A carbocation is formed when a free radical reacts with an alkene. The carbocation is subsequently attacked by the halide ion, but with fewer hydrogen atoms attached to it, the halide ion is more likely to connect to the carbon atom. This is due to the fact that the carbon atom with fewer hydrogen atoms is more electron deficient and so more likely to accept the electron from the halide ion.
A variety of factors can influence whether an addition reaction follows Markovnikov’s rule or the anti-Markovnikov’s rule. These variables include the type of reagent used, the reaction temperature, and the presence of additional compounds.
Markovnikov and Anti Marknovnikov Rule Addition Reactions Example
The Alkene Hydration Process
When alkenes are treated with certain aqueous acids (often sulfuric acid), the resultant electrophilic addition process produces alcohol. Markownikoff’s rule can predict the regioselectivity of such reactions. As a result, these reactions are known as Markovnikov reactions. In the hydration of alkenes, the H+ ion acts as an electrophile, attacking the alkene to produce a carbocation intermediate (the more stable intermediate is protonated). Water molecules then nucleophilically attack the carbocation, forming an oxonium ion that is deprotonated to yield the necessary alcohol product.
Alkene Hydroboration and Oxidation
When alkenes are treated with borane (BH3) in the presence of hydrogen peroxide or sodium hydroxide, the end result is alcohol. The boron atom acts as an electrophile in this electrophilic addition process. This reaction does not follow Markovnikov’s rule and hence qualifies as an anti-Markovnikov reaction.

 GUJCET Result 2024 Link for PDF Download...
GUJCET Result 2024 Link for PDF Download...
 BSE Odisha 10th Result 2024, Check Odish...
BSE Odisha 10th Result 2024, Check Odish...
 CUET Admit Card 2024, Check Release Date
CUET Admit Card 2024, Check Release Date















