CBSE Class 12 Chemistry Syllabus 2024-25
The Central Board of Secondary Education has published the official CBSE Class 12 Chemistry Syllabus 2024-25 for this academic year. The Class 12 Chemistry Syllabus and syllabus of other subjects create a roadmap for the board exam candidates. The knowledge they gain in the Class 12 Chemistry Syllabus 2025 helps them in later academic and professional courses. Students who study well in their classes 11 and 12th get benefits in medicine, engineering, and technology fields. Scroll to learn the details Class 12 Chemistry Syllabus, exam pattern and practical exam curriculam.
CBSE Class 12 Chemistry Syllabus 2024-25: Highlights
The CBSE Class 12 Chemistry Syllabus 2024-25 works as a guide throughout the academic year 2024-25. The Class 12 Biology paper is of a total of 70 marks. Students will get a total of 3 hours to solve the paper. Apart from this 70 marks, there is a 30 marks practical exam. Chemistry project work, viva and class records are included in these marks. Overall the Chemitry syllabus of class 12 CBSE helps to know basic facts and concepts.
Class 12 Chemistry Paper Pattern 2025
CBSE Class 12 Chemistry Theory paper consists of 10 units. We have mentioned how many periods you should attend to complete each chapter. Chapter-wise weightage is also given. As we can see the high-bearing chapters are – electrochemistry, Aldehydes, Ketones and Carboxylic Acids, and d -and f -Block Elements. Find more chapters below.
| Class 12 CBSE Chemistry Theory Paper Pattern | |||
| S.No. | Title | No. of Periods | Marks |
| 1 | Solutions | 10 | 7 |
| 2 | Electrochemistry | 12 | 9 |
| 3 | Chemical Kinetics | 10 | 7 |
| 4 | d -and f -Block Elements | 12 | 7 |
| 5 | Coordination Compounds | 12 | 7 |
| 6 | Haloalkanes and Haloarenes | 10 | 6 |
| 7 | Alcohols, Phenols and Ethers | 10 | 6 |
| 8 | Aldehydes, Ketones and Carboxylic Acids | 10 | 8 |
| 9 | Amines | 10 | 6 |
| 10 | Biomolecules | 12 | 7 |
| Total | 70 | ||
| Class 12 CBSE Chemistry Practical Paper Pattern | |
|
Evaluation Scheme for Examination
|
Marks |
| Volumetric Analysis | 8 |
| Salt Analysis | 8 |
| Content Based Experiment | 6 |
| Project Work | 4 |
| Class record and viva | 4 |
| Total | 30 |
Class 12 Chemistry Syllabus 2024-25: Theory Paper
Candidates must plan well to complete the CBSE Class 12 Chemistry Syllabus 2024-25 for theory paper on time. So that they can revise the who;e curriculum at leat two times.
Unit II: Solutions 10 Periods
Types of solutions, expression of concentration of solutions of solids in liquids, solubility of gases in liquids, solid solutions, Raoult’s law, colligative properties – relative lowering of vapour pressure, elevation of boiling point, depression of freezing point, osmotic pressure, determination of molecular masses using colligative properties, abnormal molecular mass, Van’t Hoff factor.
Unit III: Electrochemistry 12 Periods
Redox reactions, EMF of a cell, standard electrode potential, Nernst equation and its application to chemical cells, Relation between Gibbs energy change and EMF of a cell, conductance in electrolytic solutions, specific and molar conductivity, variations of conductivity with concentration, Kohlrausch’s Law, electrolysis and law of electrolysis (elementary idea), dry cell-electrolytic cells and Galvanic cells, lead accumulator, fuel cells, corrosion.
Unit IV: Chemical Kinetics 10 Periods
Rate of a reaction (Average and instantaneous), factors affecting rate of reaction: concentration, temperature, catalyst; order and molecularity of a reaction, rate law and specific rate constant, integrated rate equations and half-life (only for zero and first order reactions), concept of collision theory (elementary idea, no mathematical treatment), activation energy, Arrhenius equation.
Unit VIII: d and f Block Elements 12 Periods
General introduction, electronic configuration, occurrence and characteristics of transition metals, general trends in properties of the first row transition metals – metallic character, ionization enthalpy, oxidation states, ionic radii, colour, catalytic property, magnetic properties, interstitial compounds, alloy formation, preparation and properties of K2Cr2O7 and KMnO4.
Lanthanoids – Electronic configuration, oxidation states, chemical reactivity and lanthanoid contraction and its consequences.
Actinoids – Electronic configuration, oxidation states and comparison with lanthanoids.
Unit IX: Coordination Compounds 12 Periods
Coordination compounds – Introduction, ligands, coordination number, colour, magnetic properties and shapes, IUPAC nomenclature of mononuclear coordination compounds. Bonding, Werner’s theory, VBT, and CFT; structure and stereoisomerism, importance of coordination compounds (in qualitative analysis, extraction of metals and biological system).
Unit X: Haloalkanes and Haloarenes. 10 Periods
Haloalkanes: Nomenclature, nature of C–X bond, physical and chemical properties, optical rotation mechanism of substitution reactions.
Haloarenes: Nature of C–X bond, substitution reactions (Directive influence of halogen in monosubstituted compounds only).
Uses and environmental effects of – dichloromethane, trichloromethane, tetrachloromethane, iodoform, freons, DDT.
Unit XI: Alcohols, Phenols and Ethers 10 Periods
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only), identification of primary, secondary and tertiary alcohols, mechanism of dehydration, uses with special reference to methanol and ethanol.
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophillic substitution reactions, uses of phenols.
Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses.
Unit XII: Aldehydes, Ketones and Carboxylic Acids 10 Periods
Aldehydes and Ketones: Nomenclature, nature of carbonyl group, methods of preparation, physical and chemical properties, mechanism of nucleophilic addition, reactivity of alpha hydrogen in aldehydes, uses.
Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical and chemical properties; uses.
Unit XIII: Amines 10 Periods
Amines: Nomenclature, classification, structure, methods of preparation, physical and chemical properties, uses, identification of primary, secondary and tertiary amines.
Diazonium salts: Preparation, chemical reactions and importance in synthetic organic chemistry.
Unit XIV: Biomolecules 12 Periods
Carbohydrates – Classification (aldoses and ketoses), monosaccahrides (glucose and fructose), D-L configuration oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen); Importance of carbohydrates.
Proteins -Elementary idea of – amino acids, peptide bond, polypeptides, proteins, structure of proteins
– primary, secondary, tertiary structure and quaternary structures (qualitative idea only), denaturation of proteins; enzymes. Hormones – Elementary idea excluding structure.
Vitamins – Classification and functions.
Nucleic Acids: DNA and RNA.
CBSE Class 12 Chemistry Syllabus 2024-25: Practical Paper
CBSE Class 12 Chemistry Syllabus 2024-25 for practical paper are listed here.
Micro-chemical methods are available for several of the practical experiments. Wherever possible, such techniques should be used.
A. Surface Chemistry
(a) Preparation of one lyophilic and one lyophobic sol Lyophilic sol – starch, egg albumin and gum
Lyophobic sol – aluminium hydroxide, ferric hydroxide, arsenous sulphide.
(b) Dialysis of sol-prepared in (a) above.
(c) Study of the role of emulsifying agents in stabilizing the emulsion of different oils.
B. Chemical Kinetics
(a) Effect of concentration and temperature on the rate of reaction between Sodium Thiosulphate and Hydrochloric acid.
(b) Study of reaction rates of any one of the following:
(i) Reaction of Iodide ion with Hydrogen Peroxide at room temperature using different concentration of Iodide ions.
(ii) Reaction between Potassium Iodate, (KIO3) and Sodium Sulphite: (Na2SO3) using starch solution as indicator (clock reaction).
C. Thermochemistry
Any one of the following experiments
i) Enthalpy of dissolution of Copper Sulphate or Potassium Nitrate.
ii) Enthalpy of neutralization of strong acid (HCI) and strong base (NaOH).
iii) Determination of enthaply change during interaction (Hydrogen bond formation) between Acetone and Chloroform.
D. Electrochemistry
Variation of cell potential in Zn/Zn2+|| Cu2+/Cu with change in concentration of electrolytes (CuSO4 or ZnSO4) at room temperature.
E. Chromatography
i) Separation of pigments from extracts of leaves and flowers by paper chromatography and determination of Rf values.
ii) Separation of constituents present in an inorganic mixture containing two cations only (constituents having large difference in Rf values to be provided).
F. Preparation of Inorganic Compounds
Preparation of double salt of Ferrous Ammonium Sulphate or Potash Alum. Preparation of Potassium Ferric Oxalate.
G. Preparation of Organic Compounds
Preparation of any one of the following compounds
i) Acetanilide ii) Di -benzalAcetone iii) p-Nitroacetanilide iv) Aniline yellow or 2 – Naphthol Anilinedye.
H. Tests for the functional groups present in organic compounds:
Unsaturation, alcoholic, phenolic, aldehydic, ketonic, carboxylic and amino (Primary) groups.
I. Characteristic tests of carbohydrates, fats and proteins in pure samples and their detection in given foodstuffs.
J. Determination of concentration/ molarity of KMnO4 solution by titrating it against a standard solution of:
i) Oxalic acid,
ii) Ferrous Ammonium Sulphate
(Students will be required to prepare standard solutions by weighing themselves). K.
Qualitative analysis
Determination of one cation and one anion in a given salt.
Cation : Pb2+, Cu2+ As3+, Aℓ3+, Fe3+, Mn2+, Zn2+, Cu2+, Ni2+, Ca2+, Sr2+, Ba2+, Mg2+, NH +
Anions: (CO3)2-, S2-, (SO3)2-, (NO2)-, (SO4)2-, Cℓ-, Br-, I-, PO3- , (C2O4)2-, CH3COO-,NO –
(Note: Insoluble salts excluded)
Practicals
A. Items for Identification/Familiarity of the apparatus for assessment in practical (All experiments)
Beaker, glass rod, tripod stand, wire gauze, Bunsen burner, Whatman filter paper, gas jar, capillary tube, pestle and mortar, test tubes, tongs, test tube holder, test tube stand, burette, pipette, conical flask, standard flask, clamp stand, funnel, filter paper
Hands-on Assessment
• Identification/familiarity with the apparatus
• Odour detection in qualitative analysis
B. List of Practicals
The experiments have been divided into two sections: Section A and Section B. The experiments mentioned in Section B are mandatory.
SECTION- A
A Surface Chemistry
(1) Preparation of one lyophilic and one lyophobic sol Lyophilic sol – starch, egg albumin and gum
(2) Preparation of one lyophobic sol Lyophobic sol
– Ferric hydroxide B Chromatography
(1) Separation of pigments from extracts of leaves and flowers by paper chromatography and determination of Rf values (distance values may be provided).
C Tests for the functional groups present in organic compounds:
(1) Alcoholic and Carboxylic groups.
(2) Aldehydic and Ketonic
D Characteristic tests of carbohydrates and proteins in the given foodstuffs. E
Preparation of Inorganic Compounds- Potash Alum
SECTION-B (Mandatory)
F Quantitative analysis
(1) (a) Preparation of the standard solution of Oxalic acid of a given volume
(b) Determination of molarity of KMnO4 solution by titrating it against a standard solution of Oxalic acid.
(2) The above exercise [F 1 (a) and (b)] to be conducted using Ferrous ammonium sulphate (Mohr’s salt)
G Qualitative analysis:
(1) Determination of one cation and one anion in a given salt. Cation –NH +
Anions – CO 2-, S2-, SO 2-, Cl-, CH COO-
(Note: Insoluble salts excluded)
Note: The above practicals may be carried out in an experiential manner rather than recording observations.
Prescribed Books:
1. Chemistry Part -I, Class-XII, Published by NCERT.
2. Chemistry Part -II, Class-XII, Published by NCERT.
CBSE Class 12 Chemistry Projects
Scientific projects are included in the CBSE Class 12 Chemistry Syllabus, It involves laboratory testing and collecting information from other sources. A few suggested Projects are listed below.
• Study of the presence of oxalate ions in guava fruit at different stages of ripening.
• Study of quantity of casein present in different samples of milk.
• Preparation of soybean milk and its comparison with the natural milk with respect to curd formation, effect of temperature, etc.
• Study of the effect of Potassium Bisulphate as food preservative under various conditions (temperature, concentration, time, etc.)
• Study of digestion of starch by salivary amylase and effect of pH and temperature on it.
• Comparative study of the rate of fermentation of following materials: wheat flour, gram flour, potato juice, carrot juice, etc.
• Extraction of essential oils present in Saunf (aniseed), Ajwain (carum), Illaichi (cardamom).
• Study of common food adulterants in fat, oil, butter, sugar, turmeric power, chilli powder and pepper. Note: Any other investigatory project, which involves about 10 periods of work, can be chosen with the approval of the teacher.
CBSE Class 12 Chemistry Syllabus 2024-25 PDF
CBSE Board has released the CBSE Class 12 Chemistry Syllabus 2024-25 in a pdf format. Visit the official website at https://cbseacademic.nic.in/curriculum_2025.html to download the Class 12 biology syllabus but for your convenience, we have provided the direct pdf link to this table below.
| Link |
The CBSE Class 12 Chemistry Syllabus 2024-25 PDF is given in this article. Also check the CBSE Class 12 chemistry Syllabus for practical, projects and important books.








 CUET Chemistry Syllabus 2026, Download O...
CUET Chemistry Syllabus 2026, Download O...
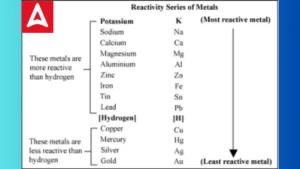 Electrochemical Series: Meaning, Table, ...
Electrochemical Series: Meaning, Table, ...
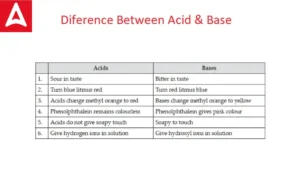 Difference Between Acid and Base, Know B...
Difference Between Acid and Base, Know B...










