Metalloids Definition
Metalloid is a type of chemical element which has a majority of properties that fall between those of metals and nonmetals, or that are a combination of both. There is no agreed-upon description of what a metalloid is, and there is disagreement about which elements fall within this category. The phrase is still used in chemistry literature despite its lack of definition.
Metalloids Example
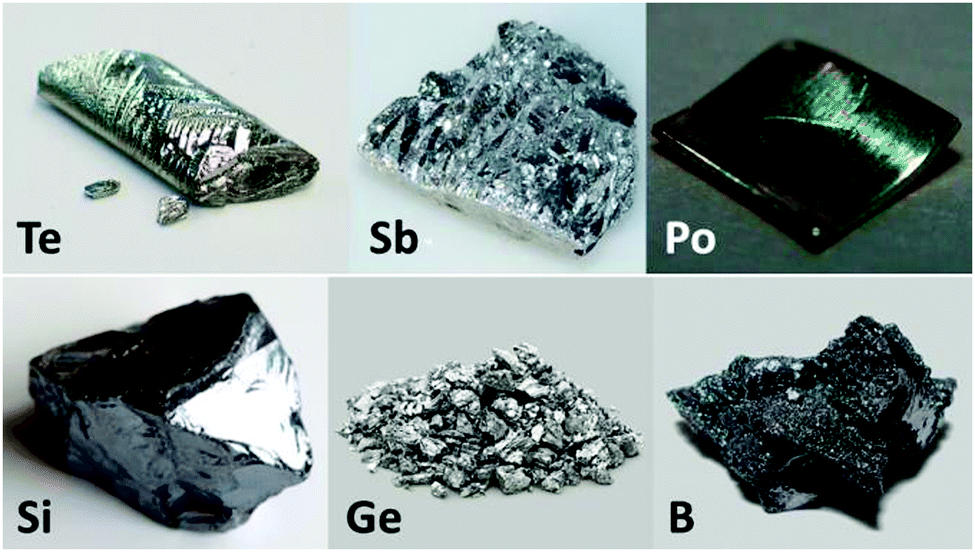
The most well-known examples of metalloids are Tellurium, Boron, Silicon, Germanium, Arsenic, Antimony, Carbon, Aluminium, Selenium, Polonium, and Astatine.
Properties of Metalloids
Following are the properties of Metalloids:
- Metalloids usually have a metallic appearance. However, these substances frequently act in a non-metallic manner.
- Physically, metalloids are solid at room temperature, brittle, and rather glossy solids.
- These substances typically exhibit moderate to strong electrical conductivity.
- Electronic band structures of metalloids are reported to resemble those of semimetals or semiconductors.
- Metalloids typically behave chemically as non-metals (in a weak way). They typically have intermediate ionisation energies and electronegativity values.
- It is known that metalloid oxides can generate amphoteric or weakly acidic oxides.
- Metalloid substances can combine to create metallic alloys.
- In essence, many of the other chemical and physical characteristics of metalloids are intermediate.
Metalloids Name
Applications of Metalloids
Following are the properties of metalloids:
- Metalloids and their compounds are frequently utilised as catalysts, flame retardants, biological agents, optical storage media, and alloys.
- Additionally, optoelectronics, semiconductors, pyrotechnics, and electronics have all been known to use metaloids.
- When it comes to the lighter metalloids, alloys created by combining transition metals are very well-represented.
- It is possible to create intermetallic compounds with boron. If the value of n is greater than 2, this element can also create alloys with certain MnB composition metals.
- In reality, ferroboron is frequently utilised to add boron to steel. Additionally, in the engineering sector, nickel-boron alloys are utilised as components in welding alloys and case hardening mixtures.
- Iron and aluminium alloys with silicon are frequently utilised in the building and auto industries.
- Germanium is well known for forming a variety of alloys, particularly those containing coinage metals.
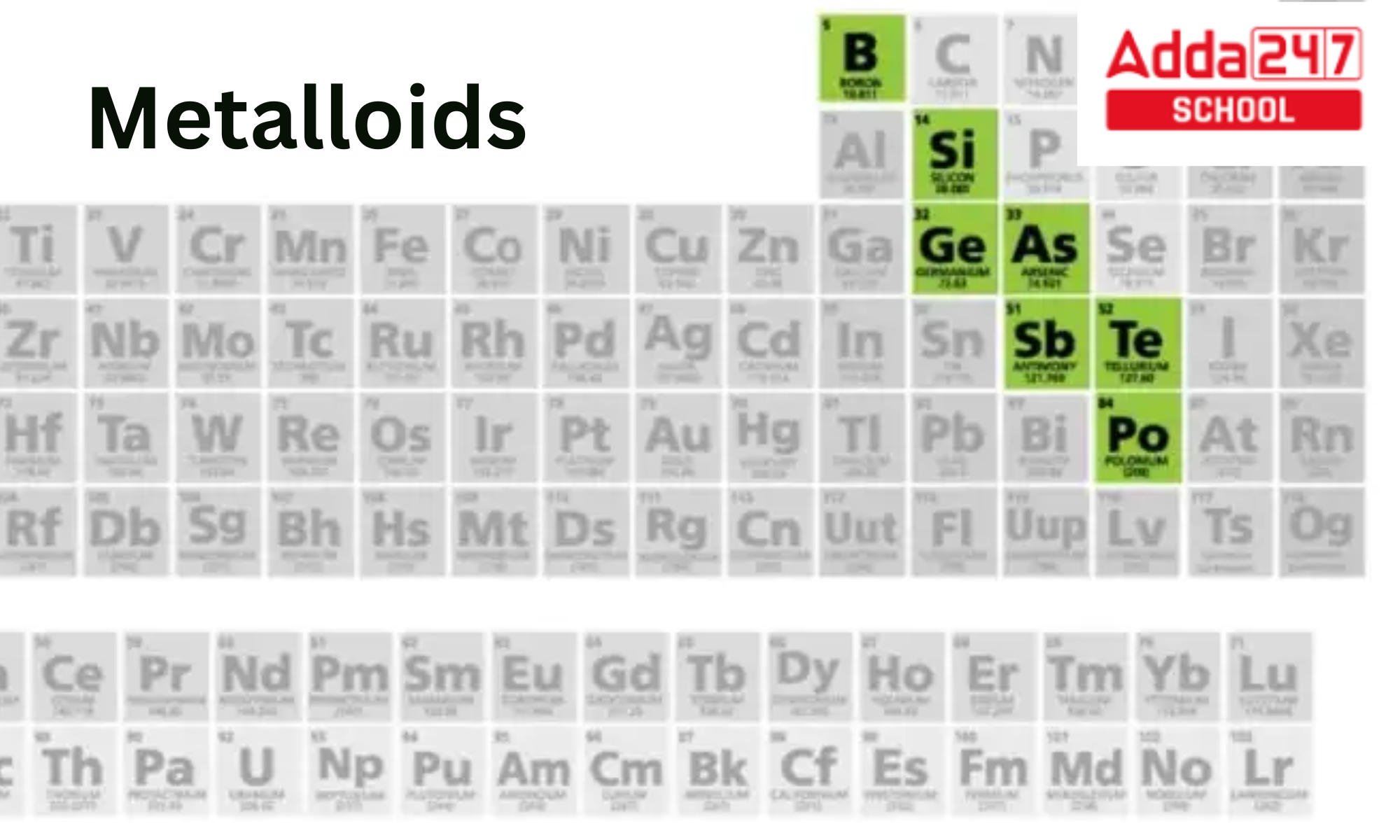
Metalloids Position in Periodic Table
On either side of the boundary between metals and nonmetals, there are metallicoids. This can be seen on certain periodic table in various forms. In general, components in the bottom left of the line exhibit increasing metallic behaviour, whereas those towards the upper right do not. The elements Li, Be, Al, Ge, Sb, and Po, which have the greatest critical temperatures for respective groups when displayed as a normal stairstep, are located close below the line.
The metalloids’ diagonal arrangement stands as an exception to the rule that similar-property elements are more likely to be found in vertical groups. Other diagonal similarities between some elements and their lower right neighbours, such lithium-magnesium, beryllium-aluminum, and boron-silicon, demonstrate a corresponding effect. According to Rayner-Canham, these parallels also exist in the carbon-phosphorus, nitrogen-sulfur, and three other d-block series.
Metalloids List
1. Boron
2. Silicon
3. Germanium
4. Arsenic
5. Antimony
6. Tellurium
7. Polonium








 CUET UG Final Answer Key 2025 Revised, D...
CUET UG Final Answer Key 2025 Revised, D...
 DU Cut off 2025, Delhi University Expect...
DU Cut off 2025, Delhi University Expect...
 OUAT Result 2025 OUT @ouat.nic.in: Check...
OUAT Result 2025 OUT @ouat.nic.in: Check...

