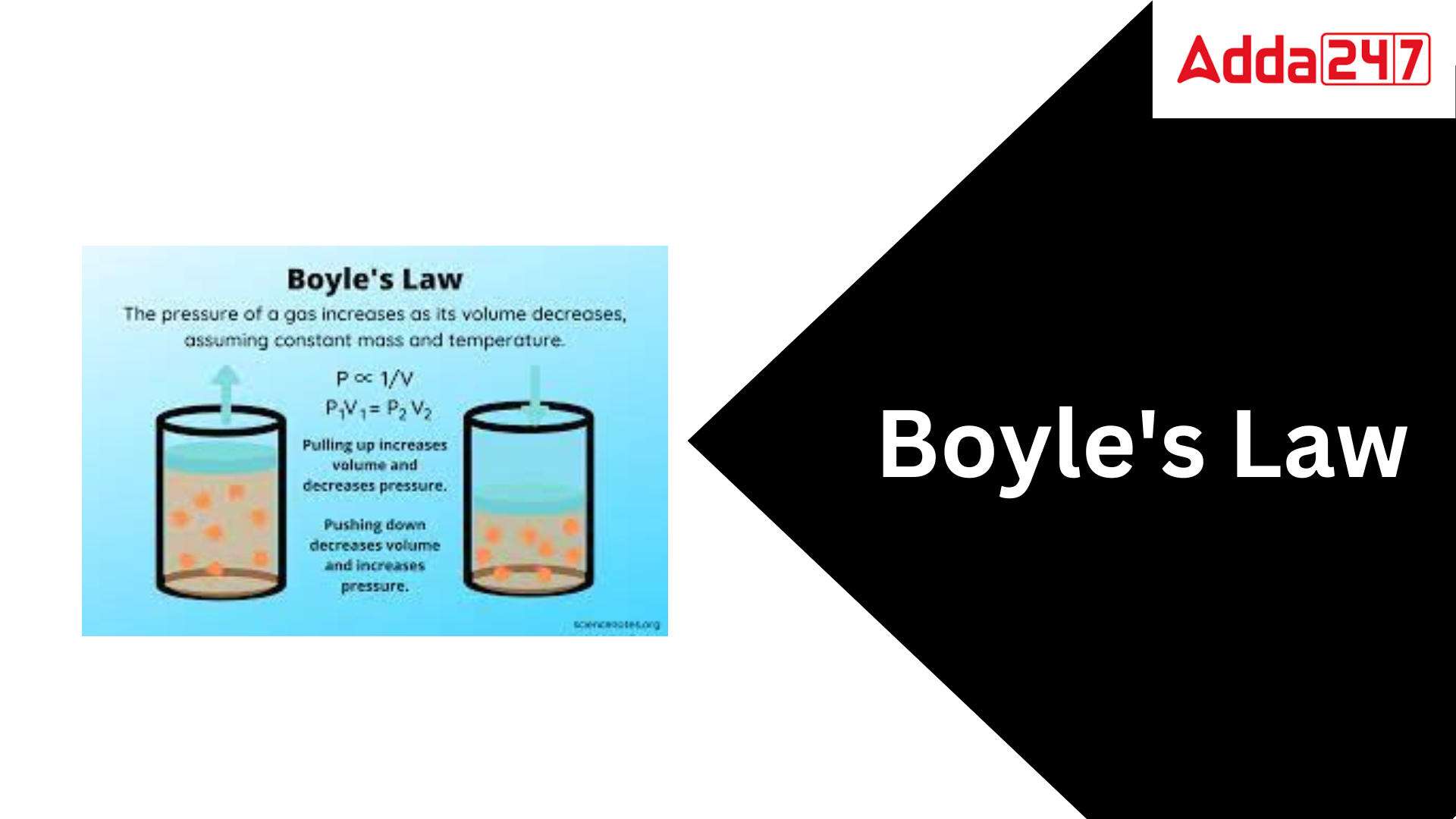
Boyle’s law: Boyle’s law is a gas law that states that the pressure exerted by a gas (with a given mass and constant temperature) is inversely proportional to its volume. In other words, as long as the temperature and amount of gas remain constant, the pressure and volume of a gas are inversely proportional to each other. Boyle’s law was proposed in 1662 by the Anglo-Irish chemist Robert Boyle.
Boyle’s law
Boyle’s law, also known as Mariotte’s law, is a gas law that asserts that the pressure and volume of a given mass of an ideal gas are inversely related if the temperature remains constant. This indicates that increasing a gas’s pressure causes its volume to drop correspondingly, and vice versa.
In 1662, the English physicist Robert Boyle proposed the law. He published his findings in The Sceptical Chymist. The law is named after him, despite the fact that it was independently discovered in 1676 by the French scientist Edme Mariotte.
Boyle’s law Formula
Boyle’s law can be expressed mathematically as follows:
P * V = k, where:
P is the gas pressure.
V is the volume of the gas, while k is a constant.
Boyle’s law is a fundamental law in physics and chemistry. It is utilized in a variety of applications, including pneumatic system design and the determination of the ideal gas constant.
Derivation of Boyle’s law formula
According to Boyle’s law, any change in the volume occupied by a gas (at constant quantity and temperature) results in a change in its pressure. In other words, the product of a gas’s starting pressure and volume equals the product of its end pressure and volume (at constant temperature and number of moles). This law is mathematically represented as follows:
P1V1 = P2V2
Where,
P1 denotes the gas’s initial pressure.
V1 denotes the gas’s initial volume.
P2 denotes the gas’s final pressure.
V2 denotes the gas’s final volume.
The pressure-volume relationship provided by Boyle’s law can be used to get this phrase. PV = k for a fixed amount of gas kept at a constant temperature. Therefore,
P1V1 = k (starting pressure * starting volume)
P2V2 = k (last pressure * last volume)
∴ P1V1 = P2V2
This equation can be used to forecast the increase in pressure imposed by a gas on the walls of its container when its volume is reduced (but its quantity and absolute temperature remain constant).
Boyle’s law Applications
Here are some examples of Everyday applications Boyle’s law :
- Blowing up a balloon: When you blow up a balloon, you increase the pressure inside the balloon. This reduces the volume of the balloon. The converse is also true. When you let the air out of a balloon, the pressure inside the balloon lowers and the volume of the balloon increases.
- Tyre pressure: The pressure within a tyre is proportional to the volume of the tyre. When you increase the pressure within a tyre, the volume of the tyre decreases. The converse is also true. When the pressure inside a tyre is reduced, the volume of the tyre increases.
- When you open a can of soda, the pressure inside the can lowers. This causes the carbon dioxide gas within the can to expand, causing the soda to foam up.
- Deep-sea diving: Divers use Boyle’s law to calculate how much oxygen they need to breathe at different depths. As you dive deeper, the pressure of the water rises, reducing the volume of air in your lungs. Divers must consider this when planning their dives.
Boyle’s law in Hindi
बॉयल का नियम (Boyle’s Law) भौतिकी में एक महत्वपूर्ण कानून है जो एक विशेष गैस के दबाव (Pressure) और आयतन (Volume) के बीच सम्बंध स्थापित करता है, यदि तापमान समान रहता है। इसे गणितीय रूप में निम्नलिखित रूप में लिखा जा सकता है:
P1 * V1 = P2 * V2
यहां, P1 और V1 गैस के प्रारंभिक दबाव और आयतन को दर्शाते हैं, और P2 और V2 परिवर्तन के बाद के दबाव और आयतन को दर्शाते हैं। जब भी गैस के दबाव और आयतन में परिवर्तन होता है और तापमान समान रहता है, तब इस नियम का प्रयोग किया जा सकता है।
Boyle’s law Examples
Example 1: A sample of gas has an initial volume of 4 liters at a pressure of 2 atmospheres. If the pressure is increased to 4 atmospheres while keeping the temperature constant, what will be the new volume?
Solution: Using Boyle’s Law, we have: P1 * V1 = P2 * V2
Given: P1 = 2 atm, V1 = 4 L, P2 = 4 atm
Now, let’s find V2 (the new volume): V2 = (P1 * V1) / P2 V2 = (2 * 4) / 4 V2 = 2 liters
So, the new volume of the gas will be 2 liters.
Example 2: A gas at a pressure of 3 atmospheres occupies a volume of 6 liters. If the volume is reduced to 2 liters while maintaining a constant temperature, what will be the new pressure?
Solution: Using Boyle’s Law, we have: P1 * V1 = P2 * V2
Given: P1 = 3 atm, V1 = 6 L, V2 = 2 L
Now, let’s find P2 (the new pressure): P2 = (P1 * V1) / V2 P2 = (3 * 6) / 2 P2 = 9 atmospheres
So, the new pressure of the gas will be 9 atmospheres.
Example 3: A gas sample has an initial pressure of 1.5 atm and a volume of 12 liters. If the volume is increased to 24 liters while the temperature remains constant, what will be the new pressure?
Solution: Using Boyle’s Law, we have: P1 * V1 = P2 * V2
Given: P1 = 1.5 atm, V1 = 12 L, V2 = 24 L
Now, let’s find P2 (the new pressure): P2 = (P1 * V1) / V2 P2 = (1.5 * 12) / 24 P2 = 0.75 atmospheres
So, the new pressure of the gas will be 0.75 atmospheres.








 MGSU Result 2025 Out, Download Maharaja ...
MGSU Result 2025 Out, Download Maharaja ...
 EMS Results 2025 OUT at gnanasangama.kar...
EMS Results 2025 OUT at gnanasangama.kar...
 How to Calculate CUET Score, Check Marks...
How to Calculate CUET Score, Check Marks...









