CBSE Class 10 Science Paper is now over. The exam was held for three hours, from 10:30 a.m. to 1:30 p.m. There were no questions determined to be outside of the syllabus. Approximately 60-70% of the questions were directly from the textbook. Students must be keen to find out how they performed following the exam. Students can refer to the Class 10 Science Answer Key 2024, which our expert faculties have explained in depth below.
We provide genuine and explanatory answers to each question answered in the Class 10 Science Examination 2024. Instead of visiting many websites, stay with us and compare your answers to the CBSE Class 10 Science Answer Key 2024 provided on this page.
Class 10 Science Answer Key 2024
CBSE Class 10 Science Answer Key 2024 provided on this page is error-free and prepared by the expert faculties of Adda247. Using the Class 10 Science Answer Key 2024, students may compare their answers to all of the questions they attempted on the exam and predict their scores. If you took your Class 10 Science Exam on March 2, 2024, you should stay tuned with us so that you do not miss the update of the Science Class 10 Answer Key 2024. Our in-house experts have also shared the Class 10 Science Exam Analysis for discussing the Science Question Paper.
Science Answer Key Class 10 2024: Overview
The CBSE Class 10 Science exam is held on 2nd March between 10:30 and 01:30. After the completion of the examination, The students can check the CBSE Class 10 Science Answer key 2024 along with Paper Analysis here. Let’s take a quick overview of the Science answer key class 10 2024.
| Particulars | Details |
| Exam Conducting Body |
Central Board of Secondary Education (CBSE)
|
| Name of Examination |
CBSE Class 10 Board Examination 2024
|
| Subject | Science |
| Mode of Exam | Offline |
| Exam Duration | 3 Hours |
| Medium of Exam | English / Hindi |
| Type of Questions |
MCQs, Short and Long Answer Type Questions
|
| Theory Marks | 80 |
| Internal Assessment | 20 |
| Total Marks | 100 |
| Passing Marks |
33% in aggregate
|
Science Class 10 Board Paper 2024: Pattern
When analyzing the science class 10 board paper 2024, the CBSE Class 10 Exam 2024 has a total of 100 marks, with the theoretical paper accounting for 80 of them and the internal evaluations accounting for the remaining 20. The CBSE Class 10 Science Question Paper 2024 has 80 marks and lasts three hours. Students have an additional 15 minutes to peruse the question paper. The CBSE science class 10 board paper 2024 consists of 39 questions divided into five sections:
| Section | Number of Questions | Marks per Question |
Weightage (in Marks)
|
| Section A: MCQs | 20 | 1 | 20 |
| Section B: Short Anwer Type Questions-I | 6 | 2 | 12 |
| Section C: Short Anwer Type Questions-II | 7 | 3 | 21 |
| Section D: Long Answer Type Questions | 3 | 5 | 15 |
| Section E: Source-Based/Case-Based Questions | 3 | 4 | 12 |
| Total Marks | 80 | ||
Class 10 Science Board Paper 2024: Analysis
In CBSE class 10 science board paper 2024 analysis, we evaluate the question paper based on errors in questions, out-of-syllabus questions, and the difficulty level of the question paper. Class 10 science board paper 2024 Analysis can help you understand the relative emphasis on different aspects of the syllabus and how difficult the exam was compared to your expectations.
According to the students, the science paper for the class 10 board examination was moderately easy to average. There were no questions determined to be outside of the syllabus. Approximately 60-70% of the questions were directly from the textbook.The CBSE Class 10 Science Board Paper Analysis by section is shared below
| Parameter |
CBSE Class 10 Science Board Paper 2024 Analysis
|
| Overall difficulty level of the paper | Easy to Moderate |
| Difficulty level of Section A |
Easy
|
| Difficulty level of Section B | Moderate |
| Difficulty level of Section C | Moderate To Tough |
| Difficulty level of Section D | Moderate To Tough |
| Difficulty level of Section E | Moderate |
| Overall expected a good score | 70+ |
| Which section was the lengthiest? | Section D |
Class 10 Science Answer Key 2024 All Sets 1, 2, 3
Science is one of the most difficult topics for many pupils. After the exam, students should look for the correct answers to each question in the CBSE Class 10 Science paper. Adda247 Experts are constantly drafting the CBSE Class 10 Science Answer Key 2024. Following the exam, you can utilize the Question Paper Solution to confirm all of the correct answers here.
Class 10 Science Answer Key 2024 Set 1: Paper Code ( 31/5/1)
SECTION A
Select and write the most appropriate option out of the four options given for each of the questions no. 1 to 20. 20×1=20
1. To balance the following chemical equation, the values of the coefficients x, y and z must be respectively :
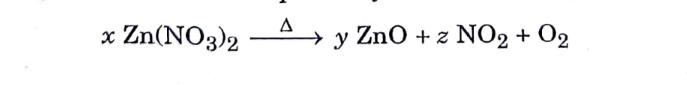 (A) 4, 2, 2
(A) 4, 2, 2
(C) 2, 2, 4
(B) 4, 4, 2
(D) 2, 4, 2
Answer: (C) 2, 2, 4
2. Which of the following is a redox reaction, but not a combination reaction?
(A) C+O2→ CO2
(C) 2 Mg + O2 → 2 MgO
(B) 2.H2 + O2 2 H₂O
(D) Fe2O3+3 CO→ 2Fe + 3 CO2
Answer:
3. The salt present in tooth enamel is:
(A) Calcium phosphate
(C) Sodium phosphate
(B) Magnesium phosphate
(D) Aluminium phosphate
Answer:
4. An aqueous solution of sodium chloride is prepared in distilled water. The pH of this solution is :
(A) 6
(C) 7
(B) 8
(D) 3
Answer:
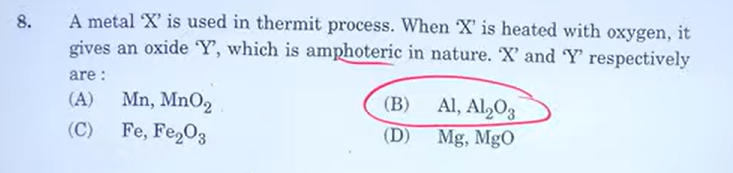
5. A metal ‘X’ is used in thermit process. When ‘X’ is heated with oxygen, it gives an oxide ‘Y’, which is amphoteric in nature. ‘X’ and ‘Y’ respectively are:
(A) Mn, MnO2
(B) Al, Al2O3
(C) Fe, Fe2O3
(D) Mg, MgO
Answer:
6. The process in which transport of soluble products of photosynthesis takes place in plants is known as:
(A) Transpiration
(C) Conduction
(B) Evaporation
(D) Translocation
Answer: (D) Translocation
7. The correct sequence of events when someone’s hand touches a hot object unconsciously:
(A) Receptors in skin → Motor neuron → Relay neuron → Sensory neuron Effector muscle in arm
(B) Receptors in skin → Relay neuron → Sensory neuron → Motor neuron Effector muscle in arm
(C) Receptors in skin → Sensory neuron → Relay neuron → Motor neuron Effector muscle in arm
(D) Receptors in skin → Sensory neuron Effector muscle in arm → Motor neuron → Relay neuron
Answer: (C) Receptors in skin → Sensory neuron → Relay neuron → Motor neuron Effector muscle in arm
8. Sense organ in which olfactory receptors are present is:
(A) Nose
(B) Skin
(C) Tongue
(D) Inner ear
Answer: (A) Nose
9.The incorrect statement about placenta is:
(A) It is a disc embedded in the uterine wall.
B) ( It contains villi on the embryo’s side of the tissue.
(C) It has a very small surface area for glucose and oxygen to from mother to the embryo.
(D) The embryo gets nutrition from the mother’s blood through it.
Answer: (C) It has a very small surface area for glucose and oxygen to from mother to the embryo.
10. Select from the following the conditions responsible for the rapid sp of bread mould on a slice of bread:
(i) Formation of large number of spores
(ii) Presence of moisture and nutrients in bread
(iv) Presence of hyphae
(A) (i) and (ii)
(B) (ii) and (iv)
(C) (ii) and (iii)
(D) (iii) and (iv)
Answer: (A) (i) and (ii)
11. How will the image formed by a convex lens be affected, if the upper half of the lens is wrapped with a black paper?
(A) The size of the image formed will be one-half of the size of the image due to complete lens.
(B) The image of upper half of the object will not be formed.
(C) The brightness of the image will reduce.
(D) The lower half of the inverted image will not be formed.
Answer: (C) The brightness of the image will reduce.
For Questions number 17 to 20, two statements are given one labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (A), (B), (C) and (D) as given below.
(A) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
(B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
(C) Assertion (A) is true, but Reason (R) is false.
(D) Assertion (A) is false, but Reason (R) is true.
17. Assertion (A): Some vegetable oils are healthy.
Reason (R): Vegetable oils generally have long unsaturated carbon chains.
Answer: (B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
18. Assertion (A): Sex of the children will be determined by what they inherit from their mother.
Reason (R): Women have XX sex chromosomes.
Answer: (D) Assertion (A) is false, but Reason (R) is true.
19. Assertion (A): Electrons move from lower potential to higher potential in a conductor.
Reason (R): A dry cell maintains electric potential difference across the ends of a conductor.
Answer: (B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
20. Assertion (A): Ozone layer protects the surface of the Earth from harmful UV radiations.
Reason (R): Chlorofluorocarbons (CFCs) are responsible for depletion of ozone layer
Answer: (B) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
SECTION B
Questions no. 21 to 26 are verry short answer type questions.
21. (a) Copper powder is taken in a china dish and heated over a burner. Name the product formed and state its colour. Write the chemical equation for the reaction involved.
OR
(b) Write chemical equation for the chemical reaction which occurs when the aqueous solutions of barium chloride and sodium sulphate react together. Write the symbols of the ions present in the compound precipitated in the reaction.
22. The melting and boiling points of carbon compounds are generally low and they are largely non-conductors of electricity. State two conclusions based on these two properties.
23.
(a) Sometimes while running, the athletes suffer from muscle cramps. Why? How is the respiration in this case different from aerobic respiration?
OR
(b) Write the other name given to lymph. State its two functions.
24. Some unicellular organisms such as Plasmodium and Leishmania differ in the manner in which they reproduce. Name and explain the reproductive process taking place in them.
25. The heat produced at a point due to concentration of sunlight by a convex lens burns a paper.
(a) Explain why it happens.
(b) Name the term (in the context of the lens used) given to the point at which the paper starts burning. What does the bright spot formed on the paper represent?
26. An electric source can supply a charge of 500 coulomb. If the current drawn by a device is 25 mA, find the time in which the electric source will be discharged completely.
SECTION C
27.
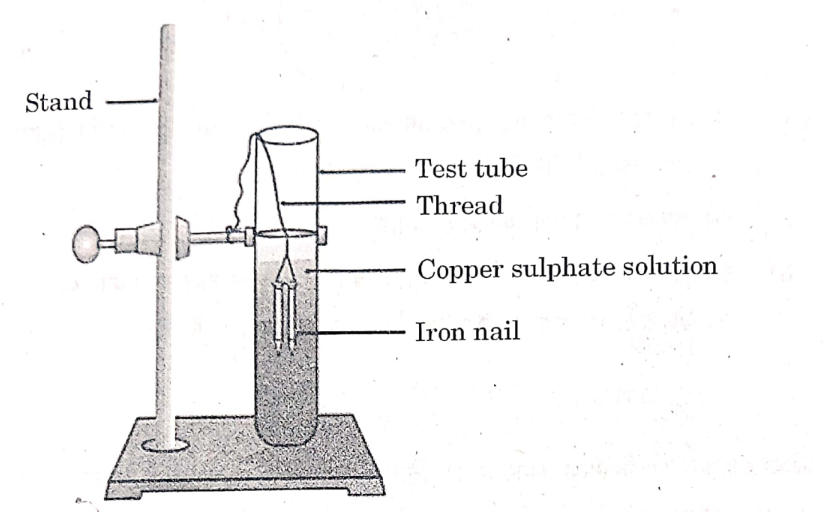
Study the experimental set-up shown in the diagram and write chemical equation for the chemical reaction involved. Name and define the type of reaction. List two other metals which can be used in place of iron to show the same type of reaction with copper sulphate solution.
28. Name the ore of mercury and state the form in which it is found in nature. Write the chemical equations along with the condition required for the reactions involved in the extraction of mercury from its ore.
29. Taking the example of any two animal hormones along with their gland of secretion, explain how these hormones help (i) in growth and development and (ii) regulate metabolism, in the body.
30. Mendel crossed pure tall pea plants (TT) with pure short pea plants (tt) and obtained F₁ progeny. When the plants of F₁ progeny were self-pollinated, plants of F₂ progeny were obtained.
(a) What did the plants of F₁ progeny look like? Give their gene combination.
(b) Why could the gene for shortness not be expressed in plants of F₁ progeny?
(c) Write the ratio of the plants obtained in F₂ progeny and state the conclusion that can be drawn from this experiment.
31. (a) Study the diagram given below and answer the questions that follow:
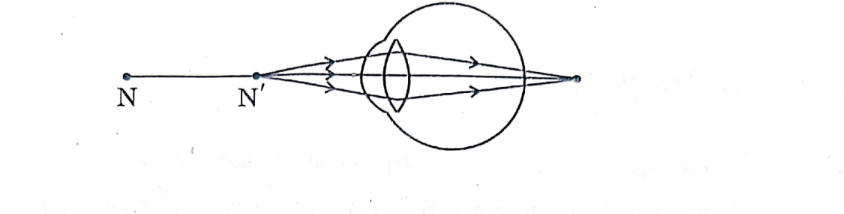
(i) Name the defect of vision depicted in this diagram stating the part of the eye responsible for this condition.
(ii) List two causes of this defect.
(iii) Name the type of lens used to correct this defect and state its role in this case.
(b) What is dispersion of white light? State its cause. Draw a diagram to show dispersion of a beam of white light by a glass prism.
32.
(a) What happens when a bundle of wires of soft iron is placed inside the coil of a solenoid carrying a steady current ? Name the device obtained. Why is it called so?
(b) Draw the magnetic field lines inside a current carrying solenoid. What does this pattern of magnetic field lines indicate?
33. Differentiate between food chain and food web. In a food chain consisting of deer, grass and tiger, if the population of deer decreases, what will happen to the population of organisms belonging to the first and third trophic levels?
Class 10 Science Answer Key 2024 Set 2: Paper Code (31/5/2)
1. Select from the following the conditions responsible for the rapid spread of bread mould on a slice of bread:
(i) Formation of large number of spores
(ii) Presence of moisture and nutrients in bread
(iii) Low temperature
(iv) Presence of hyphae
(A) (i) and (ii)
(B) (ii) and (iv)
(C) (ii) and (iii)
(D) (iii) and (iv)
Answer: (A) (i) and (ii)
2. The incorrect statement about placenta is:
(A) It is a disc embedded in the uterine wall.
(B) It contains villi on the embryo’s side of the tissue.
(C) It has a very small surface area for glucose and oxygen to pass from mother to the embryo.
(D) The embryo gets nutrition from the mother’s blood through it.
Answer: (C) It has a very small surface area for glucose and oxygen to pass from mother to the embryo.
3. An aqueous solution ‘A’ turns phenolphthalein solution pink. When another aqueous solution ‘B’ is added to the pink solution, the pink colour disappears. Now when a few drops of solution ‘A’ are added to this reaction, the mixture appears pink again. The respective changes in the nature of the solution are from:
(A) acidic basic → basic
(B) basic acidic acidic
(C) acidic basic acidic
(D) basic acidic basic
Answer: (D) basic acidic basic
5. 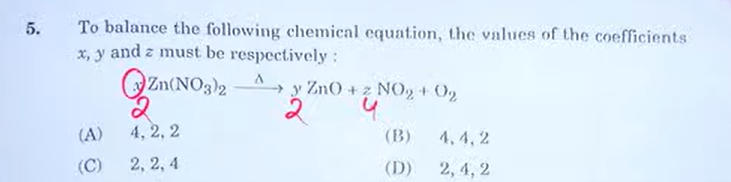
Answer: Option (C)
6. Which of the following is a redox reaction, but not a combination reaction?
(A) C+O2→ CO2 +
(B) 2H2+O2→2H₂OX
(C) 2 Mg + O2 → 2 MgO
(D) Fe2O3+3CO → 2Fe + 3 CO2
Answer: Option D
7. An aqueous solution of sodium chloride is prepared in distilled water. The pH of this solution is:
(A) 6
(C) 7
(B) 8
(D) 3
Answer: Option (C) 7
8.
11. Which one of the following is not a natural ecosystem?
(A) Pond ecosystem
(B) Grassland ecosystem
(C) Forest ecosystem
(D) Cropland ecosystem
Answer: (D) Cropland ecosystem
12. 
Answer: Option A
13. How will the image formed by a convex lens be affected, if the upper half of the lens is wrapped with a black paper?
(A) The size of the image formed will be one-half of the size of the image due to complete lens.
(B) The image of upper half of the object will not be formed.
(C) The brightness of the image will reduce.
(D) The lower half of the inverted image will not be formed
Answer:(C) The brightness of the image will reduce.
14, The phenomena of light involved in the formation of rainbow are:
(A) Refraction, reflection and dispersion
B) Refraction, dispersion and internal reflection
(C) Reflection, dispersion and internal reflection
D) Refraction, dispersion, scattering and total internal reflection
Answer: (C) Reflection, dispersion and internal reflection
15. The colour of light for which the refractive index of glass is minimum, is:
(A) Red
(C) Green
(B) Yellow
(D) Violet
Answer:(A) Red
16. The current carrying device which produces a magnetic field similar to that of a bar magnet is:
(A) A straight conductor
(C) A solenoid
(B) A circular loop
(D) A circular coil
Answer:(C) A solenoid
Class 10 Science Answer Key 2024 Set 3: Paper Code (31/5/3)
Section A
2. The incorrect statement about placenta is:
(A) It is a disc embedded in the uterine wall.
(B) It contains villi on the embryo’s side of the tissue.
(C) It has a very small surface area for glucose and oxygen to pass from mother to the embryo.
(D) The embryo gets nutrition from the mother’s blood through it.
Answer:(C) It has a very small surface area for glucose and oxygen to pass from mother to the embryo.
3. An aqueous solution ‘A’ turns phenolphthalein solution pink. When
another aqueous solution ‘B’ is added to the pink solution, the pink colour disappears. Now when a few drops of solution ‘A’ are added to this reaction, the mixture appears pink again. The respective changes in the
nature of the solution are from:
(A) acidic basic → basic
(B) basic acidic → acidic
(C) acidic basic acidic
(D) basic acidic → basic
Answer: (D) basic → acidic → basic
4. The correct sequence of events when someone’s hand touches a hot object unconsciously :
(A) Receptors in skin Motor neuron Relay neuron → Sensory neuron Effector muscle in arm
(B) Receptors in skin Relay neuron Sensory neuron → Motor neuron Effector muscle in arm
(C) Receptors in skin Sensory neuron Relay neuron→ Motor neuron Effector muscle in arm
(D) Receptors in skin Motor neuron Sensory neuron Effector muscle in arm →Relay neuron
Answer: (C) Receptors in skin Sensory neuron Relay neuron→ Motor neuron Effector muscle in arm
9. The process in which transport of soluble products of photosynthesis takes place in plants is known as:
(A) Transpiration
(B) Evaporation
(C) Conduction
(D) Translocation
Answer: (D) Translocation
10. Sense organ in which olfactory receptors are present is:
(A) Nose
(B) Skin
(C) Tongue
(D) Inner
Answer: (A) Nose
15. The colour of light for which the refractive index of glass is minimum, is:
(A) Red
(B) Yellow
(C) Green
(D) Violet
Answer:(A) Red
16. The current carrying device which produces a magnetic field similar to that of a bar magnet is: (
(A) A straight conductor
(B) A circular loop
(D) A circular coil
(C) A solenoid
Answer:(C) A solenoid
17. Assertion (A): Electrons move from lower potential to higher potential in a conductor.
Reason (R): A dry cell maintains electric potential difference across the ends of a conductor.
Answer:(B)
18. Assertion (A): Some vegetable oils are healthy.
Reason (R): Vegetable oils generally have long unsaturated carbon chains.
Answer: (B)
19. Assertion (A): Sex of the children will be determined by what they inherit from their mother.
Reason (R): Women have XX sex chromosomes.
Answer: (D)
20. Assertion (A): Green plants trap only 1% of the energy of sunlight that falls on their leaves.
Reason (R): All green plants are the producers in a food chain.
Answer:(A)
Answer Key of Science Class 10: MCQ’s Questions
Section A
Select and write the most appropriate option out of the four options given for each of the questions 1-20. There is no negative mark for the incorrect response.
1. When 2 mL of sodium hydroxide solution is added to a few pieces of granulated zinc in a test tube and then warmed, the reaction that occurs can be written in the form of a balanced chemical equation as:
(a) NaOH + Zn → NaZnO2 + H20
(b) 2NaOH + Zn → Na2ZnO2 + H2
(c) 2NaOH + Zn → NaZnO2 + H2
(d) 2NaOH + Zn → Na2ZnO2 + H2O
Answer: (b) 2NaOH + Zn → Na2ZnO2 + H2
2. Select from the following a decomposition reaction in which source of energy for decomposition is light:
(a) 2FeSO4 → Fe203+ SO2+ SO3
(b) 2H2O → 2H2+ O2
(c) 2AgBr → 2Ag + Br2
(d) CaCO3 → CaO + CO2
Answer: (c) 2AgBr → 2Ag + Br2
3. A metal and a non-metal that exists in liquid state at the room temperature are respectively :
(a) Bromine and Mercury
(b) Mercury and lodine
(c) Mercury and Bromine
(d) Iodine and Mercury
Answer: (c) Mercury and Bromine
4. Carbon compounds:
(i) are good conductors of electricity.
(ii) are bad conductors of electricity.
(iii) have strong forces of attraction between their molecules.
(iv) have weak forces of attraction between their molecules.
The correct statements are:
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (i) and (iii)
Answer: (d) (i) and (iii)
5. Consider the following compounds :
FeSO4; CuSO4; CaSO4; Na2CO3
The compound having maximum number of water of crystallisation in its crystalline form in one molecule is :
(a) FeSO4
(b) CuSO4
(c) CaSO4
(d) Na2CO3
Answer: (d) Na2CO3 ; 10 water molecules
6. Oxides of aluminium and zine are :
(a) acidic
(b) basic
(c) amphoteric
(d) neutral
Answer: (c) amphoteric; behave as both acidic and basic oxides
7. MnO2 + 4HCI → MnCl2 + 2H2O + Cl2
The reaction given above is a redox reaction because in this case
(a) MnO2 is oxidised and HCI is reduced.
(b) HCI is oxidised.
(c) MnO2 is reduced.
(d) MnO2 is reduced and HCI is oxidised.
Answer: (d) MnO2 is reduced and HCI is oxidised; MnO2 loses its oxygen to form MnCl2 and HCl loses its H to form Cl2.
8. Consider the following statements
(i) The sex of a child is determined by what it inherits from the mother.
(ii) The sex of a child is determined by what it inherits from the father.
(iii) The probability of having a male child is more than that of a female child.
(iv) The sex of a child is determined at the time of fertilisation when male and female gametes fuse to form a zygote.
The correct statements are :
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) i), (iii) and (iv)
Answer: (b) (ii) and (iv)
9. Chromosomes :
(i) carry hereditary information from parents to the next generation.
(ii) are thread like structures located inside the nucleus of an animal cell.
(iii) always exist in pairs in human reproductive cells.
(iv) are involved in the process of cell division.
The correct statements are :
(a) (i) and (ii)
(b) (iii) and (iv)
(a) (i), (ii) and (iv)
(d) (i) and (iv)
Answer: (a) (i) and (ii)
10. In a nerve cell, the site where the electrical impulse is converted into a chemical signal is known as :
(a) Axon
(b) Dendrites
(c) Neuromuscular junction
(d) Cell body
Answer: (c) Neuromuscular junction; a neuromuscular junction (or myoneural junction) is a chemical synapse between a motor neuron and a muscle fiber.
11. A stomata closes when:
(i) it needs carbon dioxide for photosynthesis.
(ii) it does not need carbon dioxide for photosynthesis.
(iii) water flows out of the guard cells.
(iv) water flows into the guard cells.
The correct reason(s) in this process is/are :
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer: (c) (ii) and (iii);
12. At what distance from a convex lens should an object be placed to get an image of the same size as that of the object on a screen?
(a) Beyond twice the focal length of the lens.
(b) At the principal focus of the lens.
(c) At twice the focal length of the lens.
(d) Between the optical centre of the lens and its principal focus.
Answer: (c) At twice the focal length of the lens;
13. The lens system of human eye forms an image on a light sensitive screen, which is called as:
(a) Cornea
(b) Ciliary muscles
(c) Optic nerves
(d) Retina
Answer: (d) Retina
Science Answer Key Class 10 2024: Paper Code 31/1/1
Check out science answer key class 10 2024 of Question Paper code 31/1/1
| Question Number | Correct Answers |
| (i) When 2 mL of sodium hydroxide solution is added to few pieces of granulated zinc in a test tube and then warmed, the reaction that occurs can be written in the form of a balanced chemical equation as : |
(b) 2NaOH + Zn → Na2ZnO2 + H2
|
| (ii) Select from the following a decomposition reaction in which source of energy for decomposition is light : |
(d) CaCO3 → CaO + CO2
|
| (iii) A metal and a non-metal that exists in liquid state at the room temperature are respectively: | (c) Mercury and Bromine |
| (iv) Carbon compounds: (i) are good conductors of electricity. (ii) are bad conductors of electricity. (iii) have strong forces of attraction between their molecules. (iv) have weak forces of attraction between their molecules. The correct statements are: |
(c) (ii) and (iv) |
| (v) Consider the following compounds : FeSO4; CuSO4; CaSO4; Na2CO3 The compound having maximum number of water of crystallisation in its crystalline form in one molecule is : |
(d) Na2CO3 |
| (vi) Oxides of aluminium and zinc are: | (c) amphoteric |
| (vii) MnO2 + 4HCl → MnCl2 + 2H2O + Cl2 The reaction given above is a redox reaction because in this case: |
(d) MnO2 is reduced and HCl is oxidised.
|
| (viii) Consider the following statements: (i) The sex of a child is determined by what it inherits from the mother. (ii) The sex of a child is determined by what it inherits from the father. (iii) The probability of having a male child is more than that of a female child. (iv) The sex of a child is determined at the time of fertilisation when male and female gametes fuse to form a zygote. The correct statements are: |
(b) (ii) and (iv) |
| (ix) Chromosomes : (i) carry hereditary information from parents to the next generation. (ii) are thread like structures located inside the nucleus of an animal cell. (iii) always exist in pairs in human reproductive cells. (iv) are involved in the process of cell division. The correct statements are: |
(d) (i), (ii), and (iv) |
| (x) In a nerve cell, the site where the electrical impulse is converted into a chemical signal is known as: | (a) Axon |
| (xi) A stomata closes when : (i) it needs carbon dioxide for photosynthesis. (ii) it does not need carbon dioxide for photosynthesis. (iii) water flows out of the guard cells. (iv) water flows into the guard cells. The correct reason(s) in this process is/are : |
(c) (ii) and (iii) |
| (xii) At what distance from a convex lens should an object be placed to get an image of the same size as that of the object on a screen? |
(c) At twice the focal length of the lens.
|
| (xiii) The lens system of human eye forms an image on a light sensitive screen, which is called as: | (d) Retina |
| (xiv) The pattern of the magnetic field produced inside a current carrying solenoid is : | (a) parallel straight lines |
| (xv) Identify the food chain in which the organisms of the second trophic level are missing: |
(c) Tiger, grass, snake, frog
|
| (xvi) In which of the following organisms, multiple fission is a means of asexual reproduction? | (d) Plasmodium |
|
Section A Part B: Assertion-Based Questions
|
|
| (i) Assertion (A): Hydrogen gas is not evolved when zinc reacts with nitric acid. Reason (R): Nitric acid oxidises the hydrogen gas produced to water and itself gets reduced. |
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
|
| (ii) Assertion (A): Accumulation of harmful chemicals is maximum in the organisms at the highest trophic level of a food chain. Reason (R): Harmful chemicals are sprayed on the crops to protect them from diseases and pests. |
(b) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
|
| (iii) Assertion (A): The rate of breathing in aquatic organisms is much faster than in terrestrial organisms. Reason (R): The amount of oxygen dissolved in water is very high as compared to the amount of oxygen in air. |
(c) Assertion (A) is true, but Reason (R) is false.
|
| (iv) Assertion (A): The rainbow is a natural spectrum of sunlight in the sky. Reason (R): Rainbow is formed in the sky when the sun is overhead and water droplets are also present in air. |
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
|
CBSE Science Question Paper 2024 PDFs
After Completion of the Exam, here we provided the CBSE Class 10 Science Question Papers 2024 PDF. Candidates may download Question Papers and analyze the Class 10 Science Answer key to calculate their expected scores.
|
CBSE Class 10 Science Question Paper 2024 PDF Download
|
|
|
CBSE Class 10 Science Question Papers 2024 -Set 1
|
Download Now |
|
CBSE Class 10 Science Question Papers 2024 Set 2
|
Download Now |
|
CBSE Class 10 Science Question Papers 2024 Set 3
|
Download Now |
| SET 31/4/3 | Download Now |
| SET 31/1/3 | Download Now |
| SET 31/2/3 | Download Now |
| SET 31/4/2 | Download Now |
Class 10 Science Paper 2024: Marking Scheme
Class 10 Science Paper 2024 comprises five sections for an overall score of 80. Check out the below mark distribution for the Class 10 Science Question Paper 2024
- Section A consists of 20 objective-type questions carrying 1 mark each.
- Section B consists of 6 Very Short questions carrying 02 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C consists of 7 Short Answer type questions carrying 03 marks each. Answers to these questions should be in the range of 50 to 80 words
- Section D consists of 3 Long Answer type questions carrying 05 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 04 marks each with sub-parts.









 CUET UG Answer Key 2025 (Provisional) Re...
CUET UG Answer Key 2025 (Provisional) Re...
 IISER Final Answer Key 2025 Out, Downloa...
IISER Final Answer Key 2025 Out, Downloa...
 OUAT Answer Key 2025 OUT @ouat.ac.in, Do...
OUAT Answer Key 2025 OUT @ouat.ac.in, Do...










