The National Testing Agency will be conducting the National Eligibility cum Entrance Test for the admission into medical courses for the academic session 2026-27 in May 2026. All the aspirants for the medical courses like MBBS and BDS must start preparing for the exam by studying all the crucial topics mentioned in the NEET Syllabus 2026. The NEET Chemistry Syllabus 2026 mentions Aldehydes, Ketones and Carboxylic Acids as one of the crucial and important topics to be covered; hence, in this article, we are sharing the NEET Notes for the Aldehydes, Ketones and Carboxylic Acids chapter. Scroll down in the article below for the important questions from this chapter.
Aldehydes, Ketones and Carboxylic Acids
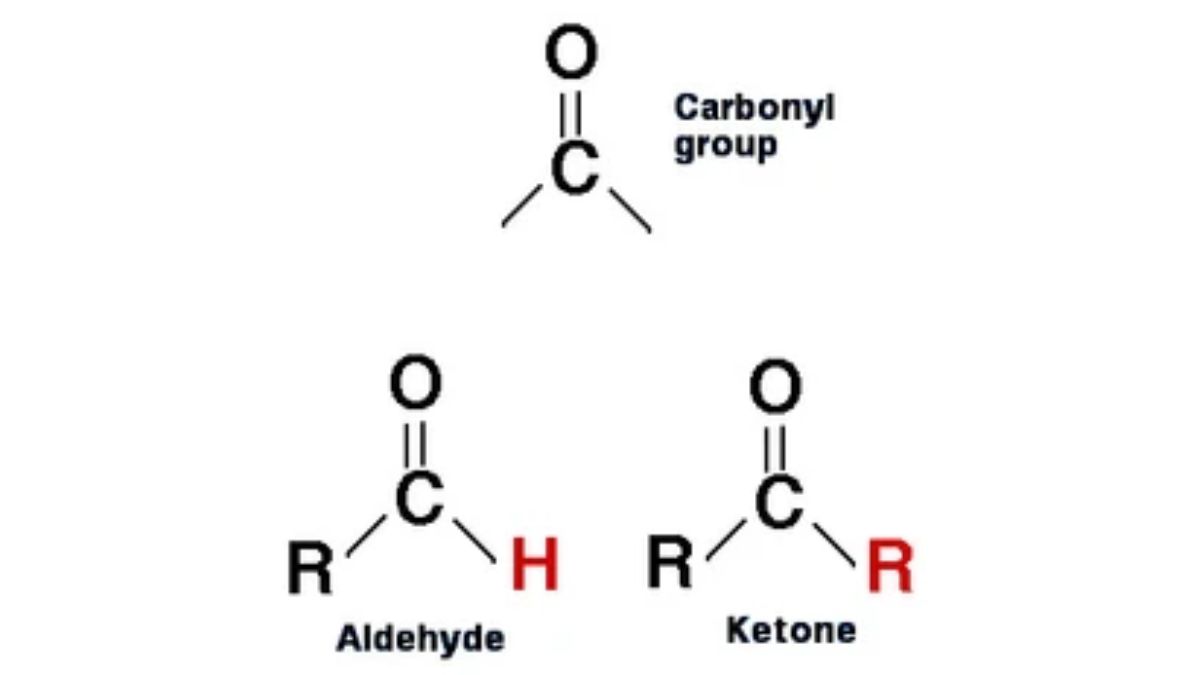
Aldehydes are organic compounds characterized by a formyl group (\(CHO\)), which is a carbonyl group (\(C=O\)) bonded to a hydrogen atom. This \(CHO\) group is located at the end of a carbon chain, distinguishing them from ketones, where the carbonyl is in the middle of the chain. Aldehydes are highly reactive, prevalent in nature, and have numerous uses in fragrances, flavorings, and industrial processes.
Ketones are molecules the body produces when it breaks down fat for energy, typically due to low carbohydrate availability. They serve as an alternative fuel source for tissues like the brain, especially during fasting, intense exercise, or for individuals with diabetes. While small amounts are normal, excessively high levels can lead to a dangerous condition called ketoacidosis.
Carboxylic acids are organic compounds with a carboxyl group (\(-COOH\)) that have acidic properties, are found widely in nature, and are used in many products, from soaps and vinegar to foods and medicines. They are polar, have strong hydrogen bonding, leading to high boiling points, and are classified as weak acids. Their reactivity allows them to form esters with alcohols and amides with amines.
Structure of the Carbonyl Group
The carbonyl carbon is sp² hybridized and forms a planar structure with a bond angle of approximately 120°. The carbon-oxygen bond is polar due to the higher electronegativity of oxygen, making the carbon electrophilic and reactive toward nucleophiles.
Preparation of Aldehydes and Ketones
- Oxidation of Alcohols:
- Primary alcohols → Aldehydes
(CH₃CH₂OH → CH₃CHO + H₂) - Secondary alcohols → Ketones
(CH₃CHOHCH₃ → CH₃COCH₃ + H₂)
- Primary alcohols → Aldehydes
- Ozonolysis of Alkenes:
- Alkene + O₃ → Ozonide → Aldehyde/Ketone
- Hydration of Alkynes:
- Terminal alkyne → Aldehyde
- Internal alkyne → Ketone
Preparation of Carboxylic Acids
- Oxidation of Aldehydes and Primary Alcohols:
- Hydrolysis of Nitriles:
- Oxidation of Alkylarenes:
Physical Properties
- Boiling Points: Aldehydes and ketones have higher boiling points than hydrocarbons due to dipole-dipole interactions, but lower than alcohols and acids (no hydrogen bonding).
- Solubility: Lower members are soluble in water due to hydrogen bonding with water molecules.
- Odour: Aldehydes often have sharp, pungent odours; ketones like acetone have sweetish odours.
Chemical Reactions of Aldehydes and Ketones
- Nucleophilic Addition Reactions:
- Addition of HCN → Cyanohydrins
- Addition of NaHSO₃ → Bisulphite addition compounds
- Addition of alcohols → Hemiacetal and Acetal formation
- Oxidation:
- Aldehydes oxidize easily to carboxylic acids.
- Ketones resist oxidation but form acids under vigorous conditions.
- Reduction:
- Reduction to alcohols using NaBH₄ or LiAlH₄.
- Aldol Condensation:
- Two molecules of aldehyde/ketone react in presence of base to form β-hydroxy aldehyde or ketone.
- Cannizzaro Reaction:
- Self oxidation and reduction of non-enolizable aldehydes (e.g., formaldehyde) in the presence of a base.
Chemical Reactions of Carboxylic Acids
- Acidic Nature:
- Carboxylic acids donate protons to form carboxylate ions.
- Strength depends on the electron-withdrawing or donating nature of substituents.
- Formation of Derivatives:
- Esters:
- Acid Chlorides:
- Amides:
- Esters:
- Decarboxylation:
- Removal of CO₂ from carboxylic acid in presence of soda lime.
Tests for Aldehydes and Ketones
- Tollen’s Test: Aldehydes give a silver mirror.
- Fehling’s Test: Aldehydes form a red precipitate of Cu₂O.
- 2,4-DNP Test: Both aldehydes and ketones form orange/yellow precipitates.
- Iodoform Test: Methyl ketones and ethanol give yellow iodoform crystals.
NEET Important Questions – Aldehydes, Ketones and Carboxylic Acids
Question 1. Write the IUPAC names of the following compounds:
(a) HCHO
(b) CH₃COCH₂CH₃
(c) C₆H₅COOH
Question 2. Distinguish between:
(a) Aldehyde and Ketone
(b) Carboxylic acid and alcohol
Question 3. How does acetone react with hydrazine?
Question 4. Write the reaction and mechanism of the aldol condensation.
Question 5. Write the mechanism of nucleophilic addition of HCN to ethanal.
Question 6. Describe the Cannizzaro reaction and name a compound that shows it.
Question 7. What is the difference between acetone and propanone?
Question 8. Why are aldehydes more reactive than ketones toward nucleophilic addition?
Question 9. Explain why formic acid is stronger than acetic acid.
Question 10. Convert:
(a) Ethanol → Ethanoic acid
(b) Acetone → Isopropanol
(c) Ethyne → Ethanal
Question 11. Write the chemical equation for the reduction of acetic acid.
Question 12. How can you distinguish between acetaldehyde and benzaldehyde?
Question 13. Explain the haloform reaction with an example.
Question 14. What is the product when benzaldehyde reacts with concentrated NaOH?
Question 15. Explain the mechanism of esterification.
Question 16. Write the steps for preparation of acetic acid from methyl cyanide.
Question 17. What are the derivatives of carboxylic acids? Give examples.
Question 18. Give a reason:
- Carboxylic acids form dimers in the vapour phase.
- Aldehydes have higher boiling points than hydrocarbons of comparable mass.
Question 19. What is Tischenko reaction? Give an example.
Question 20. Name the product formed when acetaldehyde reacts with ammonia.
Question 21. Write the structure and use of formalin.
Question 22. What happens when benzoic acid is treated with soda lime?
Question 23. How will you prepare benzaldehyde from toluene?
Question 24. How will you convert benzaldehyde into benzyl alcohol?
Question 25. Write the reaction between formic acid and PCl₅.








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









