The National Eligibility cum Entrance Test is to be conducted by the National Testing Agency at various exam centers across the nation in May 2026. All the students who are to appear for the NEET 2026 exam need to complete their exam preparations by learning all the topics mentioned in the NEET Syllabus 2026.
The Surface Chemistry is one of the most important parts of the NEET Chemistry Syllabus 2026, therefore, we have shared in the NEET Notes for the same in the article below. Scroll down in the article to check the most important questions from this chapter.
Surface Chemistry
Surface chemistry studies chemical and physical phenomena at the interface of two phases, such as solid-liquid or solid-gas. It explains why surfaces have unique properties, due to unbalanced forces on the atoms at the boundary. These phenomena include adsorption, catalysis, corrosion, and colloid formation. The surface of a substance plays a crucial role in determining its reactivity, making this topic essential for NEET aspirants to understand chemical and biological surface processes.
Adsorption
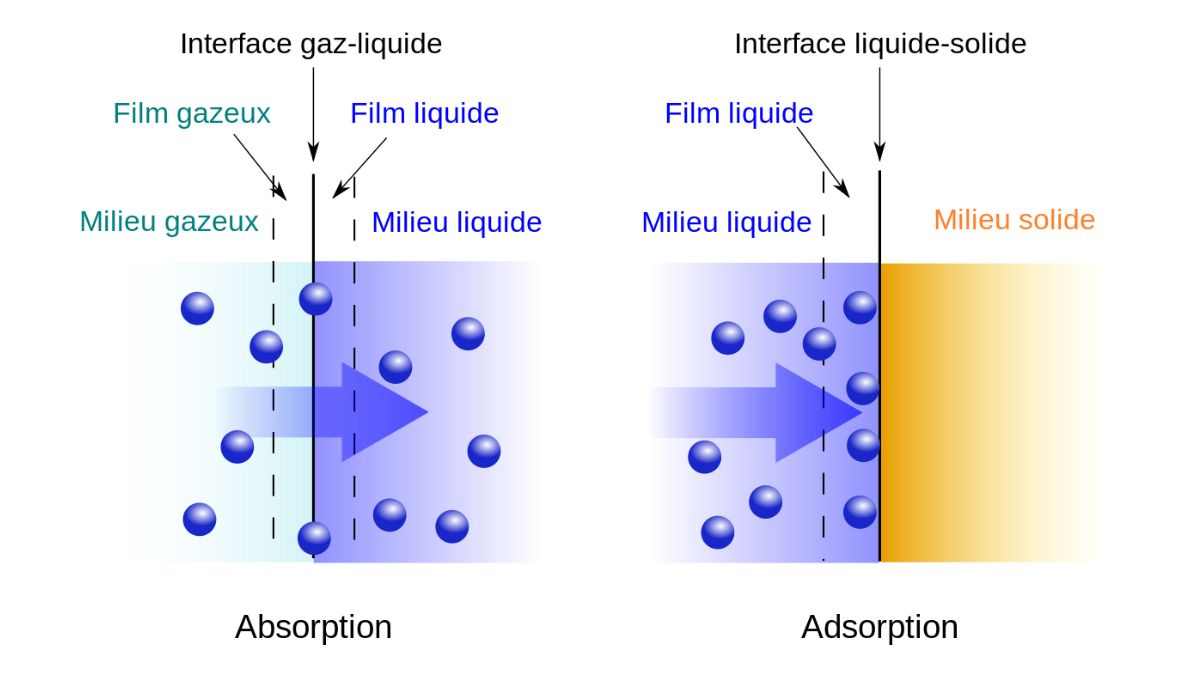
Adsorption is the process where a substance accumulates on the surface of another, driven by unbalanced forces on the surface of the solid or liquid. Molecules (adsorbate) from a gas, liquid, or dissolved solid adhere to the surface of a solid or liquid (adsorbent) without penetrating it, forming a layer on the surface. This is different from absorption, where the substance is taken into the bulk of the other material.
Types of Adsorption
Adsorption is classified into two main types: physical adsorption (physisorption) and chemical adsorption (chemisorption), based on the nature of the forces between the adsorbent and adsorbate. Physisorption involves weak van der Waals forces and does not create chemical bonds, while chemisorption involves the formation of strong chemical bonds, a process that often requires activation energy and high temperatures.
- Physical Adsorption (Physisorption):
- Involves weak van der Waals forces.
- Low enthalpy of adsorption (20-40 kJ/mol).
- Reversible process.
- Multilayer formation possible.
- Chemical Adsorption (Chemisorption):
- Involves formation of chemical bonds.
- High enthalpy of adsorption (80-240 kJ/mol).
- Irreversible.
- Unilayer formation only.
Factors Affecting Adsorption
Factors affecting adsorption include the surface area of the adsorbent, pressure of the adsorbate gas, temperature, and the nature of both the adsorbent and adsorbate. Increased surface area and pressure generally increase adsorption, while higher temperatures decrease it, especially for physical adsorption. The nature of the adsorbent and adsorbate is also critical, as some materials are more effective adsorbents than others and certain gases have a higher affinity for specific surfaces.
-
Nature of Adsorbent:
Greater surface area → higher adsorption. -
Nature of Adsorbate:
Easily liquefiable gases (NH₃, CO₂) are adsorbed more. -
Temperature:
Adsorption decreases with increase in temperature (exothermic process). -
Pressure:
For gases, adsorption increases with pressure. -
Surface Area:
Finely divided solids have larger surface area, hence higher adsorption.
Adsorption Isotherms
Adsorption isotherms describe the relationship between the amount of a substance (adsorbate) adsorbed onto a surface (adsorbent) and the adsorbate’s concentration in the fluid phase at a constant temperature and equilibrium. They are crucial for analyzing the mechanism of adsorption and are used in applications like water purification, gas separation, and catalysis. Common models, such as Langmuir and Freundlich, help to interpret experimental data and can indicate whether adsorption is monolayer and homogeneous or multilayer and heterogeneous, respectively.
Freundlich Adsorption Isotherm:
where,
-
: mass of gas adsorbed per unit mass of adsorbent
-
: pressure
-
and
: constants depending on nature of adsorbent and adsorbate
Taking log on both sides:
Graph: Straight line between
and
.
Catalysis
Catalysis is the process of increasing the rate of a chemical reaction by adding a substance called a catalyst. Catalysts work by providing an alternative reaction pathway with a lower activation energy, which is the energy barrier that reactants must overcome to become products. This results in a faster reaction without the catalyst being consumed in the process.
Types of Catalysis
The main types of catalysis are homogeneous and heterogeneous, which are classified by the physical phase of the catalyst relative to the reactants. Homogeneous catalysis occurs when the catalyst and reactants are in the same phase (e.g., both a gas or both a liquid), while heterogeneous catalysis happens when they are in different phases (e.g., a solid catalyst with liquid or gas reactants). A third category, biocatalysis, involves enzymes as biological catalysts.
-
Homogeneous Catalysis:
Catalyst and reactants are in the same phase.
Example: Oxidation of SO₂ to SO₃ in presence of NO gas. -
Heterogeneous Catalysis:
Catalyst and reactants are in different phases.
Example:-
Haber’s Process:
-
Contact Process:
-
Characteristics of Catalysis
Catalysis involves substances called catalysts that accelerate a chemical reaction without being consumed in the process. Key characteristics include increasing reaction speed by lowering activation energy, a requirement for only small quantities, specificity for certain reactions, and the ability to be recovered unchanged (though their physical form may change). Catalysts cannot initiate a reaction or change the overall equilibrium constant but help a reversible reaction reach equilibrium faster.
- Catalyst remains unchanged in mass and composition.
- Only a small quantity is required.
- Catalyst does not affect the equilibrium position.
- Highly specific in nature.
- Catalysts can be poisoned or promoted.
Colloids
Colloids are heterogeneous mixtures with dispersed particles larger than those in a true solution but too small to be seen with the naked eye, ranging from 1 to 1000 nanometers. They consist of a dispersed phase and a dispersion medium, and they exhibit the Tyndall effect, scattering light that passes through them. Examples include milk, fog, and smoke.
|
Types of Colloids (Based on Physical State):
|
|||
| Dispersed Phase | Dispersion Medium | Example | Type of Colloid |
| Solid | Solid | Coloured glass | Solid sol |
| Solid | Liquid | Paints, milk | Sol |
| Liquid | Gas | Fog, mist | Aerosol |
| Gas | Liquid | Foam, froth | Foam |
Properties of Colloidal Solutions
Colloidal solutions are heterogeneous mixtures with two phases: a dispersed phase and a dispersion medium. Key properties include the ability to scatter light (the Tyndall effect), which makes the path of light visible, and the random, continuous motion of particles called Brownian movement, which makes the solution stable and prevents particles from settling. Colloidal particles cannot be seen with the naked eye but can pass through ordinary filter paper.
- Tyndall Effect: Scattering of light by colloidal particles.
- Brownian Movement: Zig-zag motion of particles.
- Electrophoresis: Movement of colloidal particles under electric field.
- Coagulation: Settling of colloidal particles due to addition of electrolytes.
Emulsions
An emulsion is a mixture of two or more immiscible liquids, like oil and water, where tiny droplets of one liquid are dispersed throughout the other. These mixtures are unstable on their own but can be stabilized with an emulsifier, such as soap or egg yolk, which prevents the droplets from combining and separating. Common examples include milk (oil-in-water) and butter (water-in-oil), and they are used in a wide variety of products, from food and cosmetics to paint and pharmaceuticals.
Types:
- Oil in Water (O/W): Milk, vanishing cream.
- Water in Oil (W/O): Butter, cold cream.
Emulsifying Agents: Substances that stabilize emulsions, e.g., soaps, detergents.
NEET Important Questions – Surface Chemistry
Question 1. What is the effect of temperature on adsorption of gases on solids?
Question 2. Explain enzyme catalysis using the lock-and-key model.
Question 3. Distinguish between physisorption and chemisorption.
Question 4. Explain how emulsions are classified and stabilized.
Question 5. How does a catalyst increase the rate of reaction?
Question 6. Explain the term coagulation of colloids.
Question 7. What is the Tyndall effect? Explain with an example.
Question 8. Describe the preparation and properties of colloids.
Question 9. Discuss industrial applications of surface chemistry.
Question 10. What is a promoter? Give one example.
Question 11. Explain why adsorption is always exothermic.
Question 12. Describe the importance of adsorption in gas masks and chromatography.
Question 13. Explain electrophoresis and its applications.
Question 14. Define adsorption and differentiate it from absorption.
Question 15. Discuss the role of surface area and temperature in adsorption.
Question 16. Differentiate between homogeneous and heterogeneous catalysis with examples.
Question 17. State Freundlich adsorption isotherm and its significance.
Question 18. Give two examples of heterogeneous catalysis.
Question 19. Define colloids and give one example of each type.
Question 20. Explain Langmuir’s adsorption isotherm and its assumptions.








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









