The NEET 2026 exam is a national level medical entrance exam expected to be conducted by the National Testing Agency at various exam centers across the nation in May 2026. All the aspirants who are looking forward to appear for the National Eligibility cum Entrance Test for admission into the academic session 2026-27 must start preparing using their NEET Syllabus 2026 released by the NTA at its official website. The NEET Chemistry Syllabus 2026 mentions Structure of Atom as one of the important chapters, hence, in this article we have shared the NEET Notes for the Structure of Atom Chapter. Scroll down in the article to access the notes and the important questions from previous year question papers.
Structure of Atom
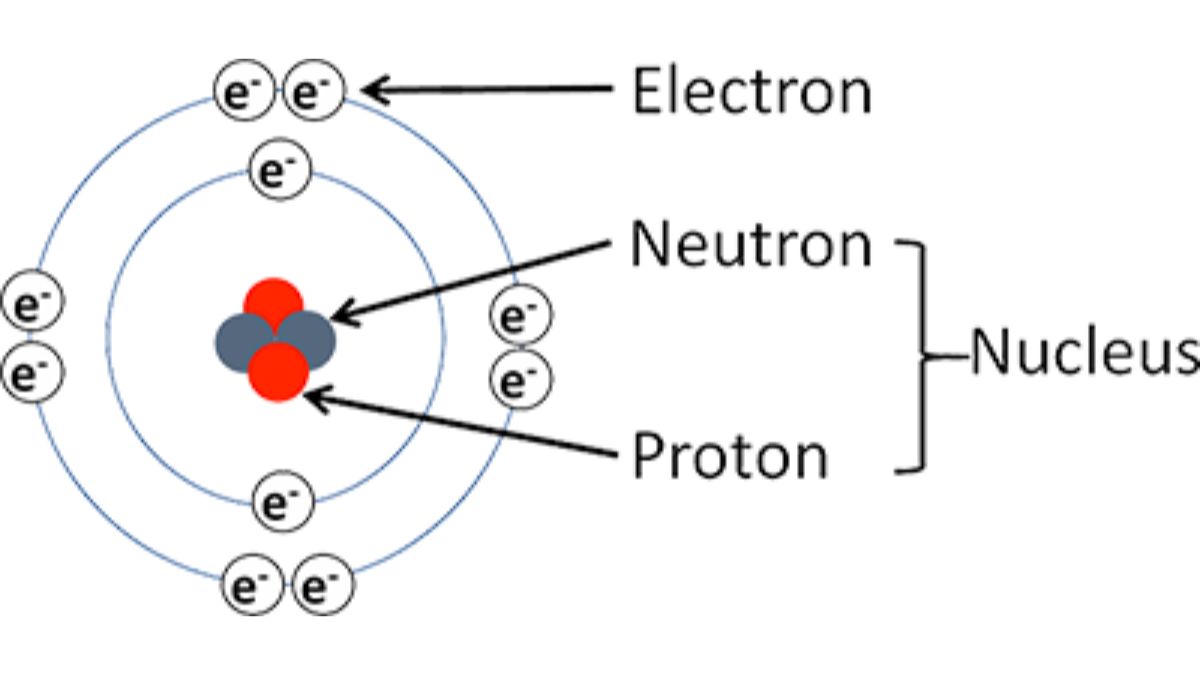
An Atom is the smallest unit of matter with retains all the chemical properties of an element. Atoms combine together to form a molecule while then combine to form solids, liquids or gases. An atom consists of a central, dense nucleus containing positively charged protons and neutral neutrons, surrounded by a cloud of negatively charged electrons. The nucleus holds nearly all of the atom’s mass, while the electrons occupy regions of space called orbitals or shells. This structure determines an atom’s identity and chemical properties.
1. Discovery of Subatomic Particles
- Electron: An electron is a negatively charged subatomic particle with a very small mass that orbits the nucleus of an atom.
- Proton: A proton is a subatomic particle with a positive electrical charge found in the nucleus of every atom.
- Neutron: A neutron is a subatomic particle with no electric charge, found in the nucleus of an atom alongside protons.
These discoveries helped in understanding that atoms are divisible and contain charged and neutral particles.
2. Atomic Models
- Thomson’s Model: Thomson’s model, also known as the “plum pudding model,” proposed that an atom is a sphere of uniform positive charge with negatively charged electrons embedded within it, like plums in a pudding.
- Rutherford’s Model: Rutherford’s model, also known as the nuclear model, describes the atom as having a tiny, dense, positively charged nucleus at its center, where most of the mass is concentrated. Negatively charged electrons orbit this nucleus in circular paths, like planets orbiting the sun.
- Bohr’s Model: Bohr’s model describes the atom as a nucleus with electrons in fixed, circular orbits, similar to a solar system but with electrostatic forces instead of gravity. Electrons can only occupy specific “stationary states” or energy levels, and they don’t radiate energy while in these orbits. Electrons can “jump” to a higher energy level by absorbing energy and emit energy (a photon) when they return to a lower level.
3. Bohr’s Model and Its Limitations
Overview:
- Electrons revolve around the nucleus in specific, circular paths called orbits or shells.
- Electrons can only exist in orbits with fixed energy values, which are numbered K, L, M, N (1, 2, 3, 4) or higher. The energy of an electron is quantized.
- Electrons can jump to a higher energy level by absorbing energy or fall to a lower one by emitting a photon of specific energy, which corresponds to a spectral line.
Limitations:
- It was unable to explain why some spectral lines were more intense then others
- It does not clarify the stark effect when the spectrum gets seperated into almost negligible lines within the sight of an electric field
- Bohr’s Model cannot be applied to multielectron atoms, even one as simple as a two electron helium atom
4. Quantum Mechanical Model of Atom
The quantum mechanical model of the atom describes electrons as waves in a probability cloud, not as particles in fixed orbits. It uses the Schrödinger equation to define orbitals, which are three-dimensional regions where electrons are most likely to be found. The model is based on probability rather than certainty, and four quantum numbers are used to define each orbital’s energy, shape, and spatial orientation.
5. Quantum Numbers
Quantum numbers are a set of four numbers that describe the unique state of an electron in an atom, specifying its energy level, the shape of its orbital, the orbital’s spatial orientation, and its spin. These numbers, known as the principal, azimuthal, magnetic, and spin quantum numbers, provide an “address” for each electron, with each electron in an atom having a distinct set of four quantum numbers.
- Principal Quantum Number (n): Energy level or shell.
- Azimuthal Quantum Number (l): Shape of the orbital (s, p, d, f).
- Magnetic Quantum Number (m): Orientation of orbital in space.
- Spin Quantum Number (s): Direction of electron spin (+½ or −½).
6. Shapes of Orbitals
The Atomic Orbitals are fainly found in four shapes, i.e., s, p, d and f. The s-orbital is spherical, the p-orbital is dumbbell-shaped, the d-orbitals have a cloverleaf shape (with one exception), and the f-orbitals are complex and multi-lobed
- s-orbital: Spherical shape.
- p-orbital: Dumbbell shape.
- d-orbital: Complex, cloverleaf shape.
- f-orbital: Complex and multi-lobed
Understanding these shapes helps visualize electron distribution.
7. Electronic Configuration
Electronic configuration is the arrangement of electrons in an atom’s shells and orbitals, showing how they are distributed in energy levels around the nucleus. This arrangement is determined by the atom’s atomic number and follows fundamental rules like the Aufbau principle, the Pauli Exclusion Principle, and Hund’s rule, which dictate how electrons fill the available orbitals from lowest to highest energy.
Example:
-
- Hydrogen: 1s¹
- Oxygen: 1s² 2s² 2p⁴








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









