The Chemistry section of the NEET 2026 Exam will comprise a total of 45 questions and to prepare for all those questions, aspirants need to study and learn all the important chapters and topics mentioned in the NEET Chemistry Syllabus 2026. One of the most important chapter that is a part of the NEET Syllabus 2026 for the Chemistry section is the P block Elements, hence, in this article, we have shared the NEET Notes for the same, guiding aspirants on what to study and where to focus on. Scroll down in the article below to access important questions from the P block Elements chapter.
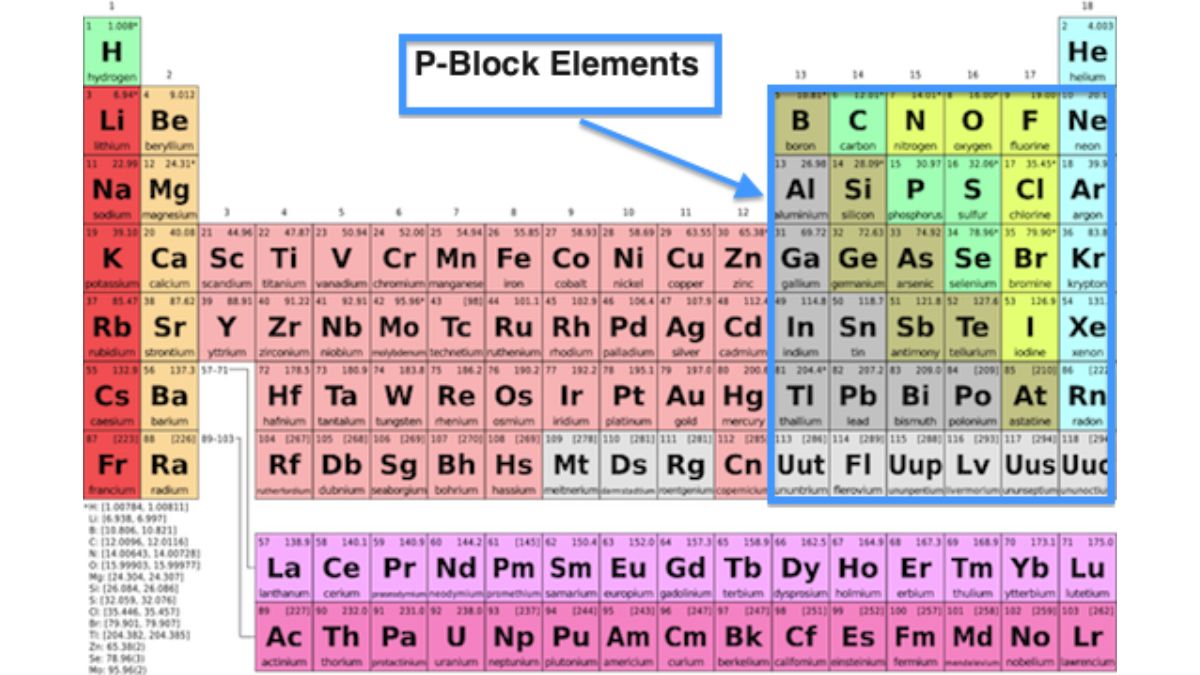
P block Elements
P-block elements are found in groups 13 through 18 of the periodic table, where valence electrons fill the outermost p-orbital. This diverse group includes metals, nonmetals, and metalloids and spans periods 2 through 7. They exhibit a wide range of properties and chemical behaviors, and include many elements essential to life, such as carbon and nitrogen, as well as technologically important materials like silicon and aluminum.
General Electronic Configuration
The general electronic configuration of p-block elements is:
- The p-subshell can accommodate a maximum of 6 electrons.
- The valence shell of these elements always contains two s-electrons and one to six p-electrons.
- The oxidation states vary due to the involvement of both ns and np orbitals, and sometimes nd orbitals in heavier elements.
Classification of P Block Elements
The p-block elements are divided into six groups:
| Classification of P Block Elements | ||
| Group | Elements | Common Name |
| 13 | B, Al, Ga, In, Tl | Boron Group |
| 14 | C, Si, Ge, Sn, Pb | Carbon Group |
| 15 | N, P, As, Sb, Bi | Nitrogen Group |
| 16 | O, S, Se, Te, Po | Oxygen Group |
| 17 | F, Cl, Br, I, At | Halogens |
| 18 | He, Ne, Ar, Kr, Xe, Rn | Noble Gases |
Important Trends in P Block Elements
Key trends in p-block elements include atomic and ionic radius decreasing across a period and increasing down a group; ionization enthalpy and electronegativity increasing across a period and decreasing down a group; and variable oxidation states, with the highest being the number of valence electrons, and the stability of these states being affected by the “inert pair effect”. Metallic character generally increases from top to bottom in a group.
- Atomic Size:
- Increases down the group due to addition of new shells.
- Decreases across the period due to increasing nuclear charge.
- Ionization Enthalpy:
- Decreases down the group.
- Increases across the period.
- Electronegativity:
- Decreases down the group.
- Increases across the period.
- Oxidation States:
- Show variable oxidation states.
- Heavier elements exhibit inert pair effect, favoring lower oxidation states.
- Metallic Character:
- Increases down the group.
- Decreases across the period.
Group 13: The Boron Family
Group 13, also known as the Boron family, includes boron (B), aluminum (Al), gallium (Ga), indium (In), thallium (Tl), and the synthetic element nihonium (Nh). These elements have an outer electron configuration of ns²np¹ and generally exhibit a trend from non-metal to metal down the group, with boron being a metalloid and the rest being metals.
- Valence configuration: ns²np¹
- Important compounds:
- Boron trichloride (BCl₃) – electron-deficient compound.
- Aluminium oxide (Al₂O₃) – amphoteric in nature.
- Uses: Aluminium is used in packaging, transportation, and electrical industries.
Group 14: The Carbon Family
Group 14, the Carbon Family, consists of carbon (C), silicon (Si), germanium (Ge), tin (Sn), and lead (Pb). These elements share a general electronic configuration of ns²np², giving them four valence electrons and a tendency to form covalent bonds. The group shows a trend from non-metallic (carbon) to metalloids (silicon, germanium) to metals (tin, lead) down the group.
- Valence configuration: ns²np²
- Allotropes of Carbon: Diamond, Graphite, Fullerenes, Graphene.
- Important Compounds:
- CO₂ (acidic oxide), SiO₂ (sand and glass material).
- Trends: Carbon forms strong covalent bonds and shows catenation (ability to form long chains).
Group 15: The Nitrogen Family
Group 15, also known as the Nitrogen Family or Pnictogens, includes nitrogen, phosphorus, arsenic, antimony, and bismuth. These elements transition from nonmetals (nitrogen, phosphorus) to metalloids (arsenic, antimony) and finally to a metal (bismuth) as you move down the group. All have the general outer electron configuration of ns²np³ and display a trend from high ionization energy and electronegativity at the top to lower values at the bottom.
- Valence configuration: ns²np³
- Key Compounds:
- NH₃ (Ammonia) – basic in nature, forms hydrogen bonds.
- HNO₃ (Nitric acid) – strong oxidizing agent.
- Trend: Oxidation states range from -3 to +5.
- Nitrogen shows anomalous behavior due to small size and high electronegativity.
Group 16: The Oxygen Family (Chalcogens)
Group 16, also known as the oxygen family or chalcogens, consists of oxygen, sulfur, selenium, tellurium, and polonium. These elements share the general valence electron configuration of ns²np⁴, giving them six valence electrons and a tendency to gain two electrons to form a -2 oxidation state. As you move down the group, there is a transition from nonmetals (oxygen, sulfur) to metalloids (selenium, tellurium) and a radioactive metal (polonium).
- Valence configuration: ns²np⁴
- Important Compounds:
- H₂O (universal solvent), O₃ (ozone) – protects from UV radiation.
- H₂S, SO₂, H₂SO₄ – industrially important.
- Trends: Metallic character increases from O to Po.
Group 17: The Halogens
Group 17, the halogens, are highly reactive nonmetals with seven valence electrons, including fluorine, chlorine, bromine, iodine, and astatine. They are characterized by their ability to form salts when combined with metals and exist as diatomic molecules
. Due to their high electronegativity and reactivity, they readily gain one electron to form halide ions (X¯) with a stable, full outer electron shell.
- Valence configuration: ns²np⁵
- Highly reactive non-metals.
- Reactivity decreases from F to I.
- Important Compounds:
- HF, HCl, HBr, HI – acid strength increases down the group.
- NaCl – essential ionic compound.
- Displacement Reaction: A more reactive halogen displaces a less reactive halogen from its compound.
Group 18: Noble Gases
Group 18, the noble gases, are a set of six elements (helium, neon, argon, krypton, xenon, and radon) known for being colorless, odorless, and highly unreactive due to their completely filled outer electron shells. This full valence shell makes them very stable, though some can form compounds under specific conditions, particularly xenon and radon. They are located on the far right of the periodic table.
- Valence configuration: ns²np⁶ (except He: 1s²)
- Monoatomic and chemically inert due to stable octet configuration.
- Uses:
- Helium – in balloons and cryogenics.
- Neon – in advertising signs.
- Argon – in light bulbs and welding.
Important Compounds of P Block
- NH₃, HNO₃, H₂SO₄, H₃PO₄ – common acids and bases.
- CO, CO₂, SiO₂ – oxides of carbon and silicon.
- BF₃, AlCl₃ – Lewis acids.
- Halogen acids (HCl, HBr, HI) – strong acids used in laboratories.
Importance of P Block Elements in NEET
- The p-block elements carry significant marks in the NEET Chemistry syllabus, especially in the Inorganic Chemistry section of Class 11 and 12 NCERT books.
- Questions based on oxidation states, periodic trends, and bonding often originate from the p-block, testing both conceptual clarity and factual knowledge.
- Many questions connect with real-life examples such as ammonia, nitric acid, ozone, and sulfuric acid, helping students relate theory with practical uses.
- Compounds of nitrogen, sulfur, and halogens play a key role in topics like acid rain, ozone layer, and pollution, which are frequent in NEET exams.
- P-block concepts are linked with chemical bonding, hybridization, and molecular geometry, helping in better understanding of physical and organic chemistry.
- Learning periodic trends (like electronegativity and metallic character) helps predict the chemical behavior of elements, an essential skill for NEET problem-solving.
- Many direct and conceptual NEET questions come from Group 13-18 topics such as boron compounds, oxides of nitrogen, and halogen acids.
- P-block topics are rich in tabular and trend-based information, making them ideal for last-minute NEET revisions and quick scoring.








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









