The National Testing Agency has announced the conduction of the National Eligibility cum Entrance Test as the process of admission into various medical courses like MBBS and BDS for the academic session 2026-27 at the registered examination centers in May 2026. All the aspirants of this exam need to start preparing for the exam by studying the subject wise NEET Syllabus 2026 available at the official portal.
In the NEET Physics Syllabus 2026, the NTA has mentioned Kinetics Theory of Gases as one of the important chapters to be studied by the aspirants. To assist all the aspirants with their preparations for this chapter we have added the NEET Physics MCQs for Kinetics Theory of Gases Chapter in the article below.
NEET Physics MCQs for Kinetics Theory of Gases Chapter
The Kinetic Theory of Gases (KTG) is a fundamental topic in NEET Physics, offering a bridge between the microscopic behavior of molecules and the macroscopic properties of gases. Questions from this chapter are generally conceptual and formula-based, making it a high-scoring section if you’ve mastered the key concepts.
This article provides a detailed guide to tackling Multiple Choice Questions (MCQs) from the KTG chapter. We’ll cover essential formulas, concepts, and a few solved examples to help you prepare effectively.
Key Concepts and Formulas
To ace the KTG questions, you must have a firm grasp of the following concepts and their associated formulas.
1. Ideal Gas Equation
An ideal gas is a theoretical gas composed of randomly moving point particles that don’t interact with each other. While real gases aren’t ideal, they behave like ideal gases at high temperatures and low pressures. The ideal gas equation is: Where:
- = Pressure
- = Volume
- = Number of moles
- = Universal gas constant ()
- = Absolute temperature (in Kelvin)
This can also be written in terms of the number of molecules (): Where:
- = Number of molecules
- = Boltzmann constant ()
- Note: , where is Avogadro’s number.
2. Kinetic Energy
The average kinetic energy of a molecule is directly proportional to its absolute temperature.
- Average Translational Kinetic Energy per molecule:
- Total Kinetic Energy for moles: This is independent of the pressure, volume, or nature of the gas. At a given temperature, all ideal gas molecules have the same average kinetic energy, regardless of their mass.
3. Degrees of Freedom
The degrees of freedom () of a molecule are the number of independent ways in which it can possess energy.
- Monatomic Gas (He, Ne, Ar): (3 translational)
- Diatomic Gas (H₂, O₂, N₂): (3 translational + 2 rotational) at room temperature. At very high temperatures, 2 vibrational degrees of freedom are also active, making .
- Polyatomic Gas (CO₂, H₂O): (3 translational + 3 rotational) for non-linear molecules.
4. Law of Equipartition of Energy
According to this law, the total energy of a system is equally distributed among its degrees of freedom. The energy associated with each degree of freedom per molecule is .
- Internal Energy () of a gas with degrees of freedom:
5. Specific Heats
- Molar Specific Heat at Constant Volume (): The heat required to raise the temperature of one mole of a gas by one Kelvin at constant volume.
- Molar Specific Heat at Constant Pressure (): The heat required to raise the temperature of one mole of a gas by one Kelvin at constant pressure.
- Ratio of Specific Heats ():
Pro-Tips for NEET Physics Kinetics Theory of Gases Chapter
Below we have added some of the most usefull and pro tips that can guide and assist aspirants to complete their preparations for the NEET 2026 Exam. Check the tips below:
- Units are Crucial: Always convert all quantities to SI units (e.g., Temperature to Kelvin, Volume to ). This is the most common mistake students make.
- Master the Postulates: Questions can be theoretical, testing your understanding of the basic assumptions of the Kinetic Theory of Gases. For instance, ideal gas molecules have negligible volume, no intermolecular forces, and perfectly elastic collisions.
- Practice with PYQs: Solving previous year’s questions (PYQs) is the single best way to prepare. They help you understand the question pattern, difficulty level, and important topics within the chapter.
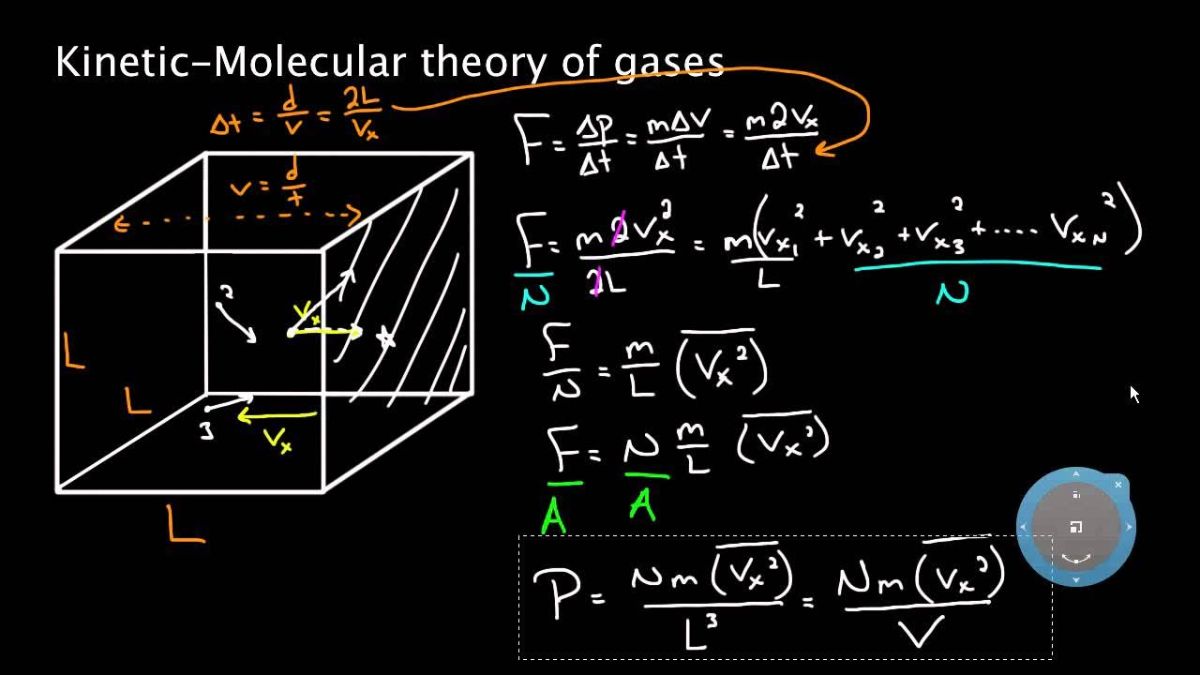
- Derive from the Basics: If you forget a formula, try to derive it from the basic principles, such as the relationship between pressure and kinetic energy () and the ideal gas equation.
Year Wise NEET Physics MCQs for Kinetics Theory of Gases Chapter
In the table listed below, we have added the year wise NEET Physics MCQs for Kinetics Theory of Gases Chapter so that all the students can start preparing accordingly. Click on the links below:
| Year Wise NEET Physics MCQs for Kinetics Theory of Gases Chapter | |
| Year | Links |
| 2014 | Click Here |
| 2015 | Click Here |
| 2016 | Click Here |
| 2017 | Click Here |
| 2018 | Click Here |
| NEET Physics Chapters | MCQ Link |
|---|---|
| Physics and Measurement | Click Here |
| Kinematics | Click Here |
| Laws of Motion | Click Here |
| Work, Energy, and Power | Click Here |
| Rotational Motion | Click Here |
| Gravitation | Click Here |
| Properties of Solids and Liquids | Click Here |
| Thermodynamics | Click Here |
| Kinetic Theory of Gases | Click Here |
| Oscillation and Waves | Click Here |
| Electrostatics | Click Here |
| Current Electricity | Click Here |
| Magnetic Effects of Current and Magnetism | Click Here |
| Electromagnetic Induction and Alternating Currents | Click Here |









 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...








