The National Eligibility cum Entrance Test is a national level medical entrance exam that test aspirants with their knowledge in Chemistry, Physics and Biology subjects. All the aspirants of the NEET 2026 exam are required to study well and learn all the topics mentioned in the NEET Syllabus 2026. One of the important chapters that is a part of the NEET Chemistry Syllabus 2026 is the Hydrocarbon; therefore, in this article, we have shared the NEET Notes for the Hydrocarbon Chapter. Scroll down in the article for the NEET Notes and the important questions from this chapter to complete exam preparations.
Hydrocarbon
Hydrocarbons are organic compounds made entirely of hydrogen and carbon atoms, forming the basis of fossil fuels like petroleum and natural gas. They are classified into four main types: alkanes (single bonds), alkenes (double bonds), alkynes (triple bonds), and aromatic hydrocarbons (ring structures like benzene). These compounds are vital for energy production (e.g., gasoline, propane) and are used to create countless industrial materials such as plastics, solvents, and synthetic fibers.
Classification of Hydrocarbons
Hydrocarbons are organic compounds of carbon and hydrogen, classified into two main groups: aliphatic and aromatic. Aliphatic hydrocarbons can be saturated (alkanes, with only single bonds) or unsaturated (alkenes with double bonds, and alkynes with triple bonds). Aromatic hydrocarbons are characterized by one or more benzene rings.
1. Alkanes (Saturated Hydrocarbons)
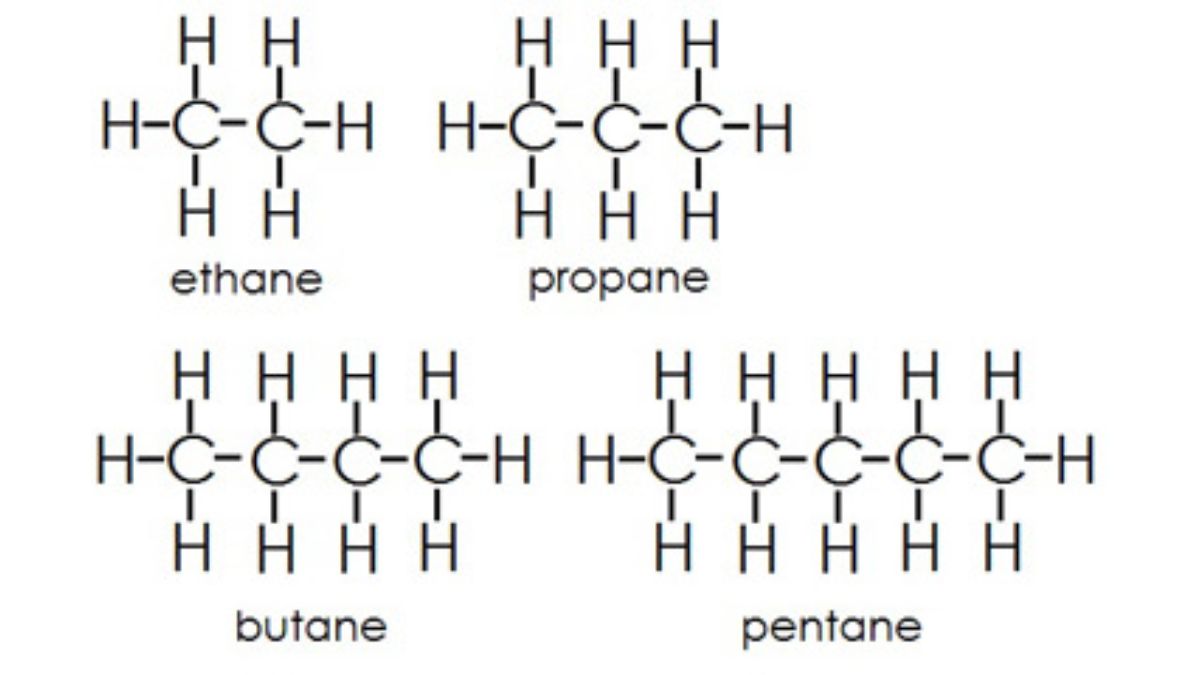
Alkanes are saturated hydrocarbons consisting only of carbon and hydrogen atoms joined by single bonds. They follow the general formula CₙH₂ₙ₊₂ and are non-polar, which makes them insoluble in water but soluble in organic solvents. Due to their saturation, alkanes are generally unreactive, and their boiling and melting points increase with molecular size.
- Contain only single bonds (C–C).
- General formula: CₙH₂ₙ₊₂
- Example: Methane (CH₄), Ethane (C₂H₆), Propane (C₃H₈)
- Structure: Straight chain, branched, or cyclic.
- Reactions:
- Combustion: Produces CO₂ and H₂O.
- Halogenation: Substitution of H by halogen (Cl₂ or Br₂) in the presence of sunlight.
2. Alkenes (Unsaturated Hydrocarbons with Double Bond)
Alkenes are unsaturated hydrocarbons with at least one carbon-carbon double bond (C=C) and the general formula CₙH₂ₙ. This double bond makes them more reactive than alkanes and gives them unique properties, such as the ability to undergo addition reactions and form geometric isomers due to restricted rotation. Alkenes are essential for producing polymers like polyethylene, antifreeze, and various other industrial chemicals.
- Contain at least one C=C double bond.
- General formula: CₙH₂ₙ
- Example: Ethene (C₂H₄), Propene (C₃H₆)
- Reactions:
- Addition reactions (e.g., Hydrogenation, Halogenation, Hydrohalogenation, Hydration)
- Polymerization: Formation of large molecules (e.g., polyethylene from ethene).
3. Alkynes (Unsaturated Hydrocarbons with Triple Bond)
Alkynes are unsaturated hydrocarbons characterized by a carbon-carbon triple bond C≡C, following the general formula CₙH₂ₙ₋₂. They are typically linear molecules, are often colorless gases or liquids, and are insoluble in water. Alkynes exhibit high reactivity due to the presence of two pi bonds in the triple bond and participate in reactions like addition, substitution, and polymerization.
- Contain at least one C≡C triple bond.
- General formula: CₙH₂ₙ₋₂
- Example: Ethyne (C₂H₂), Propyne (C₃H₄)
- Reactions:
- Addition of H₂, X₂, HX.
- Oxidation to carboxylic acids.
Nomenclature of Hydrocarbons
The nomenclature of hydrocarbons follows a system based on the IUPAC (International Union of Pure and Applied Chemistry) rules, which use a prefix for the number of carbon atoms and a suffix to indicate the type of bond. The root of the name is determined by the longest carbon chain, and a suffix is added to indicate the functional group, such as “-ane” for single bonds (alkanes), “-ene” for double bonds (alkenes), or “-yne” for triple bonds (alkynes).
- Prefix: Indicates the number of carbon atoms.
- Word root: Derived from the number of carbon atoms.
- Suffix: Indicates the type of bond:
- -ane (alkane), -ene (alkene), -yne (alkyne)
Example:
- CH₄ → Methane
- C₂H₄ → Ethene
- C₃H₆ → Propene
- C₂H₂ → Ethyne
Isomerism in Hydrocarbons
Isomerism in hydrocarbons is the phenomenon where compounds share the same molecular formula but have different atomic arrangements, leading to distinct structures and properties. The main types are structural isomerism (including chain and position isomers) and stereoisomerism (such as cis-trans isomers).
Structural Isomerism:
Structural isomerism is a type of isomerism where molecules have the same molecular formula but differ in the sequence or arrangement of their atoms, leading to different structures. Also called constitutional isomerism, this phenomenon occurs when atoms are connected in a different order, creating different compounds with different properties. Key types of structural isomerism include chain isomerism, position isomerism, and functional group isomerism.
- Chain isomerism (different carbon chain structures)
- Position isomerism (different position of functional groups or double bonds)
- Functional isomerism (different functional groups with same molecular formula)
Geometrical Isomerism:
Geometrical isomerism is a type of stereoisomerism where molecules have the same molecular formula but differ in the spatial arrangement of atoms around a double bond or in a ring structure due to restricted rotation. This restricted rotation means the atoms cannot freely rotate, leading to distinct isomers with different physical and chemical properties. These isomers are often called cis-trans isomers, where “cis” means on the same side and “trans” means on opposite sides of the double bond or ring.
- Seen in alkenes due to restricted rotation around double bonds (cis–trans or E–Z isomerism).
Important Reactions of Hydrocarbons
The important reactions of hydrocarbons include combustion, addition (for unsaturated hydrocarbons like alkenes and alkynes), and substitution (for saturated hydrocarbons like alkanes and aromatic compounds). Other key reactions are halogenation (a type of substitution or addition), cracking, and pyrolysis.
1. Combustion:
-
All hydrocarbons burn in air to give carbon dioxide and water.
2. Substitution Reaction (Alkanes):
3. Addition Reaction (Alkenes and Alkynes):
4. Polymerization:
5. Aromatic Substitution:
Important NEET Question – Hydrocarbon
Question 1: Define hydrocarbons and mention their classification.
Question 2: What is the hybridization of carbon atoms in ethane, ethene, and ethyne?
Question 3: Explain the mechanism of halogenation of methane.
Question 4: Explain hydrogenation of alkenes using a catalyst.
Question 5: Identify the type of isomerism shown by CH₃CH=CHCH₃ and CH₃CH₂CH=CH₂.
Question 6: What is Markovnikov’s rule? Give one example.
Question 7: Write the product formed when ethyne reacts with bromine water.
Question 8: How many structural isomers are possible for C₅H₁₂?
Question 9: What are aromatic hydrocarbons? Give two examples.
Question 10: Draw all possible structural isomers of C₄H₁₀ and name them.
Question 11: Why do alkynes show more reactivity towards addition reactions than alkenes?
Question 12: Write the structure of cis- and trans- but-2-ene.
Question 13: Write the IUPAC name and structural formula of CH₃–CH₂–C≡CH.
Question 14: What is the general formula for alkanes, alkenes, and alkynes?
Question 15: How can ethene be converted into ethane?
Question 16: What is polymerization? Give one example.
Question 17: Differentiate between alkanes, alkenes, and alkynes based on bonding.
Question 18: Explain anti-Markovnikov addition with an example.
Question 19: What is the product when benzene reacts with nitric acid in presence of concentrated H₂SO₄?
Question 20: What happens when toluene undergoes chlorination in presence of light?
Question 21: Why are aromatic compounds more stable than expected?
Question 22: Describe ozonolysis reaction of alkenes.
Question 23: Why does benzene undergo electrophilic substitution instead of addition?
Question 24: Why are alkanes called saturated hydrocarbons?
Question 25: Why are alkanes chemically less reactive than alkenes and alkynes?
Question 26: Explain the difference between addition and substitution reactions.
Question 27: Why does alkyne produce a sooty flame on combustion?
Question 28: What type of reaction is observed in benzene-addition or substitution? Why?
Question 29: Write a chemical equation for the combustion of propane.
Question 30: Write the IUPAC names of:
(a) CH₃CH=CH₂
(b) CH₃C≡CH








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









