Electrochemistry is a crucial chapter in the NEET Chemistry syllabus, focusing on the interplay between chemical reactions and electrical energy. Understanding key principles in electrochemistry can help aspirants tackle various questions related to this chapter, which is important for the exam. Below is an in-depth guide to the major concepts, topics, and essential questions covered in this chapter.
Electrochemistry
Electrochemistry is the study of the relationship between electrical and chemical energy, focusing on reactions that involve the transfer of electrons (redox reactions). It encompasses two main types of cells: galvanic (voltaic) cells, which convert chemical energy into electrical energy through spontaneous reactions (like in batteries), and electrolytic cells, which use electrical energy to drive non-spontaneous chemical changes. Key concepts include electrodes (anode and cathode), the Nernst Equation, and standard electrode potential, which are used to understand and predict reaction behavior.
Ahead, we have shared an overview of the key topics covered in the Electrochemistry chapter so that all the aspirants can have a better understanding of all of them.
1. Redox Reactions
-
Oxidation: Loss of electrons
Example: Zn → Zn²⁺ + 2e⁻ -
Reduction: Gain of electrons
Example: Cu²⁺ + 2e⁻ → Cu -
Redox reactions are the foundation of all electrochemical processes.
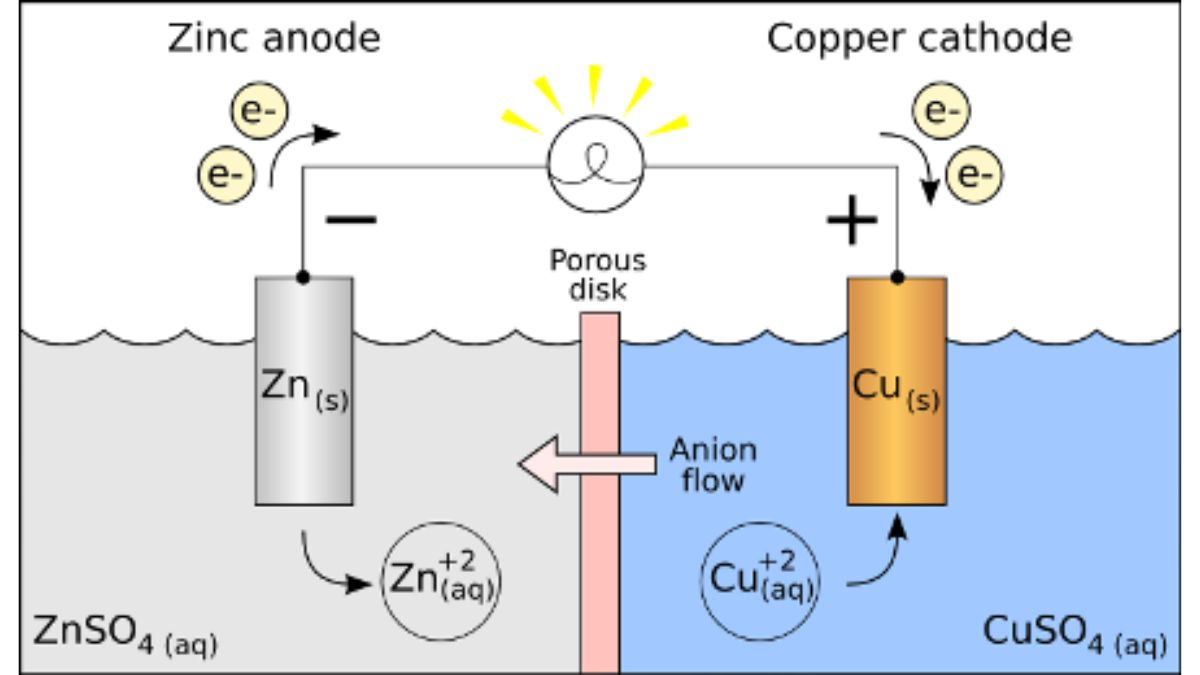
2. Electrochemical Cells
Electrochemical cells are systems that convert chemical energy into electrical energy or vice versa.
Types of Cells:
-
Galvanic (Voltaic) Cell – Chemical energy → Electrical energy
Example: Daniell Cell (Zn-Cu cell)-
Anode (−): Site of oxidation (Zn → Zn²⁺ + 2e⁻)
-
Cathode (+): Site of reduction (Cu²⁺ + 2e⁻ → Cu)
-
Salt Bridge: Maintains electrical neutrality.
-
-
Electrolytic Cell – Electrical energy → Chemical energy
Example: Electrolysis of molten NaCl
3. EMF of a Cell (Electromotive Force)
-
EMF is the potential difference between two electrodes when no current flows through the cell.
-
Cell EMF = E°(Cathode) − E°(Anode)
4. Standard Electrode Potential (E°)
-
The potential of an electrode when all species are in their standard states (1 M, 1 atm, 25°C).
-
Measured with respect to Standard Hydrogen Electrode (SHE) having E° = 0.00 V.
5. Nernst Equation
The Nernst equation relates the cell potential under non-standard conditions:
Where:
-
R = Gas constant (8.314 J/mol·K)
-
T = Temperature (K)
-
n = Number of electrons involved
-
F = Faraday’s constant (96500 C/mol)
-
Q = Reaction quotient
At 25°C (298 K),
6. Conductance and Conductivity
-
Conductance (G): Reciprocal of resistance (R).
-
Specific Conductivity (κ): Conductance of 1 cm³ of a solution.
-
Molar Conductivity (Λₘ): Conductivity of all ions from 1 mole of electrolyte.
7. Kohlrausch’s Law of Independent Migration of Ions
At infinite dilution, the molar conductivity of an electrolyte is the sum of the individual ionic conductivities of its ions.
8. Electrolysis
Electrolysis is the process of using electrical energy to drive a non-spontaneous chemical reaction.
Faraday’s Laws of Electrolysis:
-
First Law: Mass (m) of the substance deposited is directly proportional to the charge (Q) passed.
-
Second Law: When the same amount of charge passes through different electrolytes, the masses deposited are proportional to their equivalent weights.
NEET Important Questions – Electrochemistry Chapter
Question 1. Determine the number of Faradays required to deposit 27 g of Al. (Atomic mass = 27)
Question 2. Why does molar conductivity of a weak electrolyte increase rapidly with dilution?
Question 3. A current of 3 A is passed for 10 minutes through AgNO₃ solution. Find the mass of Ag deposited. (E = 107.9)
Question 4. What is meant by standard electrode potential?
Question 5. Explain why EMF of a cell decreases with time.
Question 6. What happens at the anode and cathode during electrolysis of molten NaCl?
Question 7. Write the reactions occurring at each electrode in the electrolysis of water.
Question 8. What is the function of a salt bridge in a galvanic cell?
Question 9. Differentiate between galvanic and electrolytic cells.
Question 10. State Kohlrausch’s Law and its applications.
Question 11. How is the Nernst equation useful in calculating electrode potentials?
Question 12. How much copper (in grams) will be deposited when a current of 2 A is passed through CuSO₄ solution for 30 minutes? (E = 63.5)
Question 13. Calculate the molar conductivity of 0.01 M NaCl solution if κ = 0.001412 S cm⁻¹.
Question 14. In a Daniell cell, if [Zn²⁺] = 0.1 M and [Cu²⁺] = 1.0 M, find the EMF at 298 K.
Question 15. Define oxidation and reduction in terms of electron transfer.
Question 16. What is the standard hydrogen electrode and why is it used?
Question 17. Calculate the EMF of the cell:
Zn | Zn²⁺ (1 M) || Cu²⁺ (1 M) | Cu
Given: E°(Zn²⁺/Zn) = –0.76 V, E°(Cu²⁺/Cu) = +0.34 V.
Question 18. Calculate the cell potential at 298 K for the cell:
Zn | Zn²⁺ (0.01 M) || Cu²⁺ (1 M) | Cu.
Question 19. For the cell:
Ag | Ag⁺ (0.1 M) || Cu²⁺ (1 M) | Cu,
calculate E_cell if E°(Ag⁺/Ag) = +0.80 V and E°(Cu²⁺/Cu) = +0.34 V.
Question 20.Using Kohlrausch’s law, calculate Λₘ°(NaCl) if
λ°(Na⁺) = 50.1 S cm² mol⁻¹ and λ°(Cl⁻) = 76.3 S cm² mol⁻¹.








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









