The NEET Chemistry Syllabus 2026 is now live at the official website of the National Testing Agency, so that all the aspirants who are looking forward to appearing for the NEET 2026 exam can access it and start their preparations accordingly. The NEET Syllabus 2026 for Chemistry subject contains Coordination Compound as one of the main chapters; hence, it is crucial for all the aspirants to study and learn all the key topics covered in this chapter. To assist aspirants with their exam preparations, we have shared the NEET Notes for the Coordination Compound chapter in the article below.
Coordination Compound
Coordination compounds consist of a central metal atom or ion bonded to surrounding ligands (molecules or ions) via coordinate covalent bonds. For the NEET exam, key topics include the structure and bonding, types of ligands, nomenclature, isomerism, and theories like Valence Bond Theory (VBT) and Crystal Field Theory (CFT) to explain properties such as color and magnetism. These compounds are vital in various applications, including biochemistry (e.g., hemoglobin, chlorophyll), catalysis, and analytical chemistry.
To assist aspirants with their exam preparations, we have shared the overview of all the important topics covered in the Coordination Compound chapter:
Key Terms and Definitions
-
Coordination Entity:
The part of the compound enclosed in square brackets, consisting of the central atom and its ligands.
Example: In, the coordination entity is
.
-
Central Metal Atom/Ion:
Usually a transition metal that accepts electron pairs from ligands.
Example: Co in.
-
Ligands:
Ions or molecules that donate a lone pair of electrons to the central atom.-
Monodentate: Binds through one donor atom (e.g., NH₃, Cl⁻).
-
Bidentate: Binds through two donor atoms (e.g., ethylenediamine).
-
Polydentate: Binds through more than two donor atoms (e.g., EDTA⁴⁻).
-
-
Coordination Number:
The number of ligand donor atoms bonded to the central atom.
Example: In, coordination number = 6.
-
Oxidation Number:
The apparent charge on the central metal ion after accounting for ligand charges.
Example: In, oxidation number of Co = +3.
Nomenclature of Coordination Compounds
- Cation is named before anion.
- Ligands are named alphabetically before the metal.
- Prefixes (di-, tri-, tetra-) are used for multiple identical ligands.
- The oxidation state of the metal is written in Roman numerals in parentheses.
Examples:
→ Hexaamminecobalt(III) chloride
→ Tetraaquacopper(II) sulfate
Isomerism in Coordination Compounds
Isomerism occurs when compounds have the same formula but different arrangements.
Structural Isomerism:
- Ionization Isomerism: Exchange of ligands between inner and outer spheres.
Example:
and - Hydrate Isomerism: Different numbers of water molecules inside/outside the coordination sphere.
- Linkage Isomerism: Ligands bind through different donor atoms.
Example:
(nitro) and (nitrito).
Stereoisomerism:
- Geometrical Isomerism: Different spatial arrangements (cis/trans).
Example:
– cis and trans forms. - Optical Isomerism: Non-superimposable mirror images (enantiomers).
Bonding in Coordination Compounds
Werner’s Theory (1893):
- Central metal exhibits primary valence (oxidation number) and secondary valence (coordination number).
- Ligands satisfy secondary valences and are directed in specific geometries.
Valence Bond Theory (VBT):
- Explains hybridization of orbitals to form coordination bonds.
- Example:
– hybridization → octahedral geometry.
Crystal Field Theory (CFT):
- Explains splitting of d-orbitals in the presence of ligands.
- Strong field ligands (CN⁻, CO) → large splitting → low spin.
- Weak field ligands (H₂O, F⁻) → small splitting → high spin.
Applications of Coordination Compounds
- Biological Importance:
Hemoglobin (Fe), Chlorophyll (Mg), Vitamin B₁₂ (Co) are coordination complexes. - Industrial Uses:
Catalysts like Wilkinson’s catalyst
. - Analytical Chemistry:
Estimation of metal ions using complexometric titrations with EDTA. - Medicinal Uses:
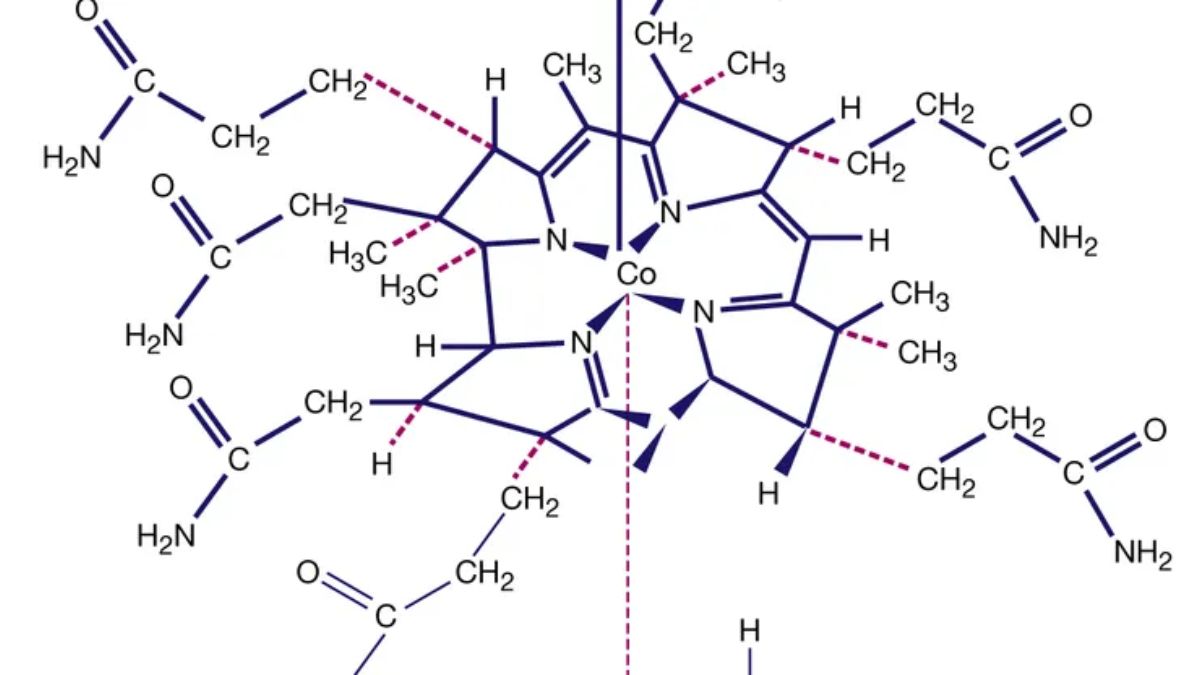
Cisplatin (
) is used in cancer treatment.
Importance of Coordination Compound NEET Notes
All the aspirants who are looking forward to appear for the NEET Exam must use NEET Notes for the Coordination Compound as one of the key sources for their exam preparations. Check the benefits of using Coordination Compound NEET Notes ahead:
- High-Weightage NEET Topic: Coordination Compounds are among the most frequently asked chapters in NEET Chemistry, often contributing direct and conceptual questions that can boost overall scores.
- Builds Inorganic Chemistry Foundation: This topic helps students strengthen their understanding of chemical bonding, hybridization, and oxidation numbers-key fundamentals for mastering inorganic chemistry.
- Improves Conceptual Clarity: The notes explain complex ideas like isomerism, valence bond theory, and crystal field theory in a simplified manner, making it easier for students to visualize molecular structures.
- Enhances Problem-Solving Skills: Students learn to calculate oxidation numbers, identify geometries, and name coordination compounds systematically-essential for both NEET and board exams.
- Connects Theory with Real-World Applications: Understanding coordination compounds provides insight into biological systems like hemoglobin and industrial catalysts, linking classroom learning to practical chemistry.
- Simplifies Nomenclature and Structural Understanding: The notes help students memorize and apply IUPAC naming conventions and recognize geometrical and optical isomers quickly during exams.
- Useful for Quick Revision: Concise and organized NEET notes allow for fast revision before exams, helping recall definitions, formulas, and examples efficiently.
- Foundation for Higher Studies: A strong grasp of coordination chemistry is beneficial for advanced studies in chemistry, biochemistry, and medical sciences, making it academically and professionally valuable.
NEET Important Questions – Coordination Compound
Question 1. What is a ligand? Classify ligands based on their denticity with examples.
Question 2. Explain the importance of coordination compounds in biological systems.
Question 3. Describe the biological significance of coordination compounds with examples.
Question 4. What are ambidentate ligands? Give two examples.
Question 5. Write the electronic configuration of the central metal ion in [Fe(CN)6]3−[Fe(CN)_6]^{3-}[Fe(CN)6]3−.
Question 6. Define linkage isomerism and give an example.
Question 7. Identify the type of isomerism shown by [Co(NH3)5Br]SO4[Co(NH_3)_5Br]SO_4[Co(NH3)5Br]SO4 and [Co(NH3)5SO4]Br[Co(NH_3)_5SO_4]Br[Co(NH3)5SO4]Br.
Question 8. How does CFT explain the stability of low-spin and high-spin complexes?
Question 9. Explain the difference between primary valence and secondary valence according to Werner’s theory.
Question 10. What are the postulates of Crystal Field Theory?
Question 11. Which of the following is diamagnetic and why? [NiCl4]2−[NiCl_4]^{2-}[NiCl4]2− or [Ni(CN)4]2−[Ni(CN)_4]^{2-}[Ni(CN)4]2−.
Question 12. Explain why transition metals form colored complexes.
Question 13. What is the role of EDTA in complexometric titrations?
Question 14. Write the IUPAC name of [Co(en)2Cl2]Cl[Co(en)_2Cl_2]Cl[Co(en)2Cl2]Cl.
Question 15. Discuss the role of ligands in determining the stability of coordination compounds.
Question 16. What is meant by chelation? Give one example of a chelating ligand.
Question 17. Explain the hybridization and geometry of [Ni(CN)4]2−[Ni(CN)_4]^{2-}[Ni(CN)4]2−.
Question 18. What are strong field and weak field ligands? Give examples of each.
Question 19. What is the difference between a double salt and a coordination compound? Give one example of each.
Question 20. Define a coordination compound and give two examples.
Question 21. Why does [Fe(H2O)6]3+[Fe(H_2O)_6]^{3+}[Fe(H2O)6]3+ appear yellow in color while [Fe(CN)6]3−[Fe(CN)_6]^{3-}[Fe(CN)6]3− is colorless?
Question 22. Explain ionization isomerism with a suitable example.
Question 23. Name the complex compound used in the treatment of cancer and give its chemical formula.
Question 24. How many geometric isomers are possible for [Co(NH3)4Cl2]+[Co(NH_3)_4Cl_2]^+[Co(NH3)4Cl2]+?
Question 25. Explain crystal field splitting in octahedral and tetrahedral complexes with diagrams.
Question 26. Explain the terms monodentate, bidentate, and polydentate ligands with suitable examples.
Question 27. Arrange the following ligands in increasing order of field strength: I−I^-I−, Br−Br^-Br−, CN−CN^-CN−, NH3NH_3NH3.
Question 28. Write the IUPAC name of [Cr(NH3)5Cl]Cl2[Cr(NH_3)_5Cl]Cl_2[Cr(NH3)5Cl]Cl2.
Question 29. What are optical isomers? Give an example of a complex that shows optical isomerism.
Question 30. What is meant by the coordination number of a metal ion? Give one example each for coordination number 4 and 6.
Question 31. Why are most transition metal complexes colored?
Question 32. Write the formula and name of the complex ion formed when Cu²⁺ reacts with excess NH₃.
Question 33. Determine the oxidation number of Fe in [Fe(CN)6]4−[Fe(CN)_6]^{4-}[Fe(CN)6]4−.
Question 34. Why do coordination compounds show variable magnetic properties?
Question 35. Calculate the oxidation state of cobalt in [Co(en)3]Cl3[Co(en)_3]Cl_3[Co(en)3]Cl3.
Question 36. State the limitations of Valence Bond Theory.
Question 37. Differentiate between inner orbital and outer orbital complexes with examples.
Question 38. Explain geometrical isomerism with an example of an octahedral complex.
Question 39. Predict the number of unpaired electrons in [FeF6]3−[FeF_6]^{3-}[FeF6]3− and [Fe(CN)6]3−[Fe(CN)_6]^{3-}[Fe(CN)6]3−.
Question 40. Which of the following complexes will show optical isomerism:
(a) [Co(en)3]3+[Co(en)_3]^{3+}[Co(en)3]3+
(b) [Co(NH3)6]3+[Co(NH_3)_6]^{3+}[Co(NH3)6]3+
(c) [Cr(H2O)4Cl2]+[Cr(H_2O)_4Cl_2]^+[Cr(H2O)4Cl2]+








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









