The NEET Syllabus 2026 released by the National Testing Agency at its official website contains chapters from the Chemistry, Biology and Physics subjects. All the aspirants of the NEET 2026 exam must ensure that they learn all the chapters mentioned in the syllabus. One such important chapter that is a crucial part of the NEET Chemistry Syllabus 2026 is the Classification of Elements and Periodicity in Properties NEET Notes, therefore, in this article, we have shared the NEET Notes for this chapter. Scroll down in the article below to find NEET Notes and access the important questions that help you complete exam preparations.
Classification of Elements and Periodicity in Properties
Classification of Elements and Periodicity in Properties is the organization of elements in the periodic table by increasing atomic number, creating a system where recurring patterns in properties-like atomic size, ionization energy, and electronegativity be observed and predicted. The periodic table arranges elements into 7 horizontal periods and 18 vertical groups, with similar chemical properties arising from similar valence electron configurations in the same group.
Historical Development of the Periodic Table
The development of the Periodic table has been a tale of limitations and innovations. Below, we have mentioned the different stage that explains the historical development of the Periodic Table. Check the pointers below:
-
Dobereiner’s Triads (1829):
Johann Dobereiner grouped elements in triads where the atomic mass of the middle element was approximately the average of the other two (e.g., Li, Na, K).
Limitation: Applicable only to a few elements. -
Newlands’ Law of Octaves (1866):
John Newlands arranged elements in increasing order of atomic mass and found that every eighth element had similar properties.
Limitation: Failed after calcium and ignored undiscovered elements. -
Mendeleev’s Periodic Table (1869):
Dmitri Mendeleev arranged elements based on atomic mass and left gaps for undiscovered elements like gallium and germanium.
Merit: Predicted undiscovered elements correctly.
Limitation: Position of isotopes and some anomalies were unexplained. -
Modern Periodic Law (Moseley, 1913):
Henry Moseley rearranged elements based on atomic number (Z) instead of atomic mass.
Modern Periodic Law: The physical and chemical properties of elements are periodic functions of their atomic numbers.
Modern Periodic Table
The Modern Periodic Table organizes chemical elements by increasing atomic number in rows called periods and columns called groups. Elements in the same group have similar chemical properties, while elements change from metals to nonmetals across a period. The table is divided into four blocks, s, p, d, and f, and includes separate rows at the bottom for lanthanides and actinides.
Structure of the Periodic Table
- Periods: 7 horizontal rows
- Groups: 18 vertical columns
- Blocks: Based on the type of orbital filled
- s-block: Groups 1–2
- p-block: Groups 13–18
- d-block: Groups 3–12 (Transition elements)
- f-block: Lanthanides and Actinides
Periodic Properties and Their Trends
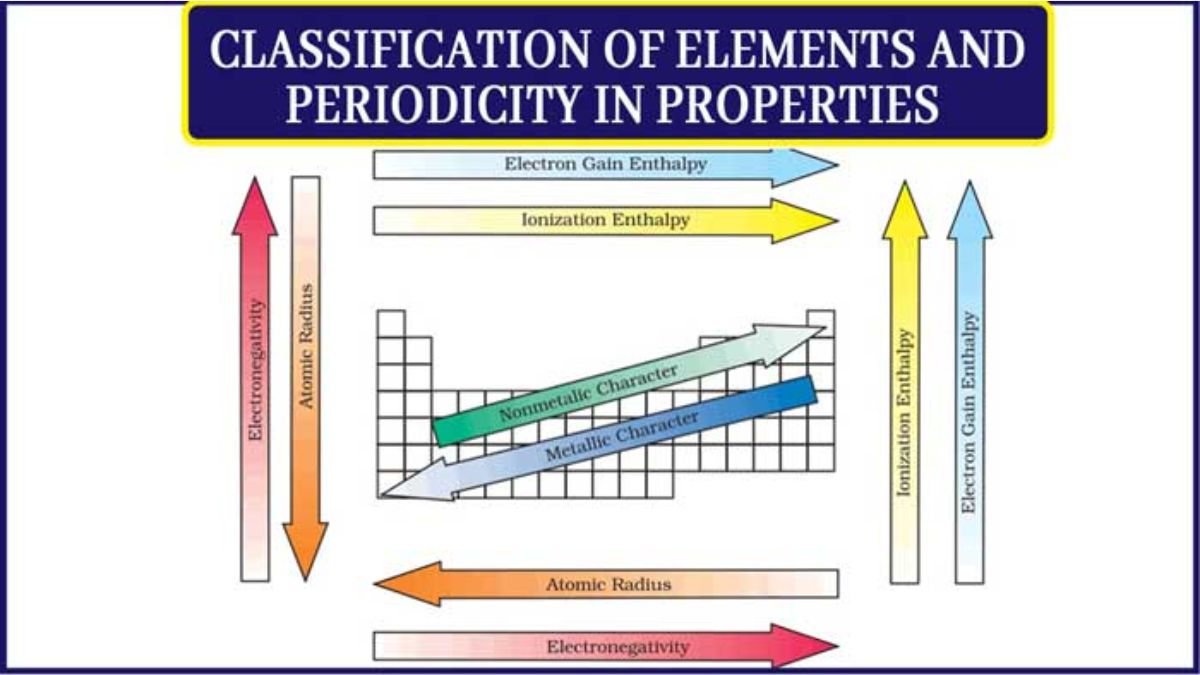
Periodic properties are characteristics of elements that show a gradual change across periods and down groups in the periodic table due to recurring electronic configurations. Key trends include atomic radius, ionization energy, electron affinity, electronegativity, and metallic character. In general, atomic radius and metallic character decrease from left to right across a period and increase down a group, while ionization energy, electron affinity, and electronegativity increase from left to right and decrease down a group.
Atomic Radius
Atomic radius is a measure of an atom’s size, defined as the average distance from its nucleus to the outermost electron shell. It is not a fixed value and depends on the type of bond an atom forms, so it’s often determined by measuring half the distance between the nuclei of two bonded, identical atoms. On the periodic table, atomic radius generally increases as you move down a group and decreases as you move from left to right across a period.
-
Trend:
- ↓ Down a group: Increases (due to new shells added)
- → Across a period: Decreases (due to increasing nuclear charge)
Ionization Enthalpy
Ionization enthalpy is the energy required to remove an electron from a gaseous atom in its ground state. It increases across a period of the periodic table and decreases down a group due to factors like nuclear charge, atomic size, and the shielding effect. Ionization enthalpy is a measure of how strongly an atom holds onto its electrons, influencing an element’s reactivity and chemical properties, such as its metallic or non-metallic character.
-
Trend:
- ↓ Decreases down a group
- → Increases across a period
Electron Gain Enthalpy
Electron gain enthalpy is the energy change that occurs when an electron is added to a neutral gaseous atom, forming a negative ion (X(g) + e¯→ X¯(g)). It indicates how strongly an atom can attract an extra electron, with a negative value meaning energy is released (exothermic) and a positive value meaning energy is absorbed (endothermic). Key factors influencing it are nuclear charge, atomic size, and electronic configuration.
-
Trend:
- ↓ Becomes less negative down a group
- → Becomes more negative across a period
Electronegativity
Electronegativity is the measure of an atom’s tendency to attract a shared pair of electrons in a chemical bond. It helps determine the type of bond formed-ionic, polar covalent, or nonpolar covalent-and is measured on a unitless scale, with fluorine being the most electronegative element. Electronegativity trends show it increases across a period (left to right) and decreases down a group (top to bottom) on the periodic table.
-
Trend:
- ↓ Decreases down a group
- → Increases across a period
Metallic and Non-Metallic Character
Metallic and non-metallic characters are two opposite sets of properties of elements, distinguished by their tendency to lose or gain electrons, respectively. Metals are typically lustrous, malleable, ductile, and good conductors of heat and electricity, while non-metals are often dull, brittle, and poor conductors. Metallic character increases down a group and decreases across a period, whereas non-metallic character increases across a period and decreases down a group.
-
Metallic Character: Tendency to lose electrons.
↓ Increases down a group
→ Decreases across a period -
Non-Metallic Character: Tendency to gain electrons.
Opposite of metallic character.
Valency
-
Definition: The combining capacity of an element.
Determined by the number of valence electrons.
Valency first increases from 1 to 4 across a period and then decreases back to 0.
Anomalous Properties of the First Element
The first element in each group of the periodic table (e.g., Li, Be, B, C, N, O, F) shows anomalous properties compared to other members of its group due to its small atomic size, high electronegativity, and absence of d-orbitals in its valence shell. These factors lead to unique behaviors, such as a high charge density, higher ionization energy, and different types of bonding or reactivity.
- Small atomic and ionic size
- High ionization enthalpy
- Absence of d-orbitals
Periodic Trends and Chemical Reactivity
Periodic trends explain how chemical reactivity changes based on an element’s position in the periodic table, driven by its electron configuration and atomic structure. Reactivity is high for elements on the far left and top right, which readily lose or gain electrons, respectively. Across a period, reactivity generally decreases and then increases as metallic character gives way to non-metallic character; down a group, reactivity increases for metals and decreases for non-metals.
- Metals: Reactivity increases down a group (e.g., alkali metals).
- Non-metals: Reactivity decreases down a group (e.g., halogens).
- Noble Gases: Exhibit minimal reactivity due to complete valence shells.
NEET Important Questions – Classification of Elements and Periodicity in Properties
Question 1. State Modern Periodic Law and explain its importance.
Question 2. Why did Mendeleev leave gaps in his periodic table?
Question 3. Define periodicity in properties.
Question 4. Why does atomic radius decrease across a period?
Question 5. Explain why ionization enthalpy increases across a period.
Question 6. Why does fluorine have the highest electronegativity?
Question 7. What are Dobereiner’s triads? Give one example.
Question 8. Why are noble gases chemically inert?
Question 9. Explain the difference between atomic radius and ionic radius.
Question 10. What are anomalous properties of the first element of each group?
Question 11. Arrange the following in increasing atomic radius: N, O, F.
Question 12. Arrange the following in increasing ionization enthalpy: Li, Na, K.
Question 13. Compare the electron gain enthalpy of chlorine and fluorine.
Question 14. Which has higher Zeff: Na or Mg? Explain.
Question 15. Write the electronic configuration of an element with atomic number 17.
Question 16. Which element has higher metallic character – Na or Mg?
Question 17. Arrange F, Cl, Br, I in the order of increasing atomic radius.
Question 18. Why does oxygen have a lower ionization energy than nitrogen?
Question 19. Which element in the second period shows maximum electronegativity?
Question 20. Explain why the reactivity of alkali metals increases down the group.








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









