The National Eligibility cum Entrance Test, which is a national level medical entrance exam, is to be conducted by the National Testing Agency at various exam centers across the nation in May 2026. All the aspirants who are looking forward to appearing for admission into the medical courses like MBBS and BDS must start preparing for the exam by reading and learning all the topics and chapters mentioned in the NEET Syllabus 2026.
In the article below, we have added the NEET Notes for the Chemical Thermodynamics chapter so that all the aspirants can read them and complete their preparations by practicing using the important questions shared in the article.
Chemical Thermodynamics
Chemical thermodynamics is the study of energy changes, particularly heat and work, in chemical reactions and physical transformations. It uses the principles of the laws of thermodynamics to determine the feasibility, spontaneity, and equilibrium of chemical processes, and to quantify the energy involved. Key concepts include internal energy, enthalpy (H), entropy (S), and Gibbs free energy (G), which help predict the direction and extent of a reaction.
Basic Concepts of Thermodynamics
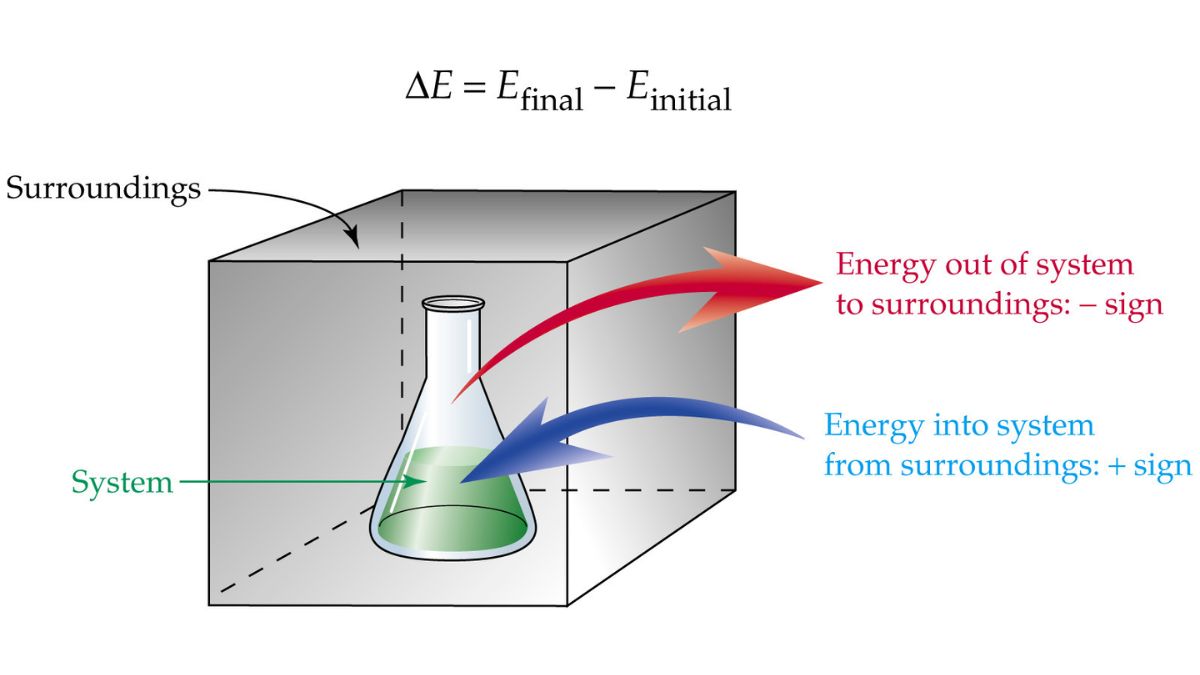
Thermodynamics is the study of heat, work, temperature, and energy, and their relation to matter. Key concepts include the distinction between a system (the part of the universe being studied) and its surroundings, and the three types of systems: isolated (no energy or matter exchange), closed (only energy exchange), and open (both energy and matter exchange). Other core ideas involve the four laws of thermodynamics, which govern energy and the feasibility of processes, and the concepts of heat transfer, work, and entropy.
System and Surroundings: A thermodynamic system is the specific part of the universe under study, and everything else is the surroundings. Types of Systems:
- Isolated: Exchanges neither energy nor matter with its surroundings.
- Closed: Can exchange energy with its surroundings, but not matter.
- Open: Can exchange both energy and matter with its surroundings.
First Law of Thermodynamics
The First Law of Thermodynamics is the law of conservation of energy, which states that energy cannot be created or destroyed, only converted from one form to another. It establishes a relationship between heat, work, and the internal energy of a system, with the change in a system’s internal energy equal to the heat added to the system minus the work done by the system (ΔU=Q-W).
Where:
- ΔU = Change in internal energy
- q = Heat added to the system
- w = Work done on the system
Sign Conventions:
- Heat absorbed by the system (+q)
- Heat released by the system (–q)
- Work done on the system (+w)
- Work done by the system (–w)
Work, Heat, and Internal Energy
Heat, work, and internal energy are core concepts in thermodynamics, describing energy transfer and storage. Internal energy is the total kinetic and potential energy of a system’s atoms and molecules. Heat is energy transferred due to a temperature difference, while work is energy transferred through a force acting over a distance. The relationship is summarized by the first law of thermodynamics, which states that the change in a system’s internal energy (ΔU) is equal to the heat added to the system (Q) plus the work done on the system (W), or (ΔU=Q+W). Note that conventions may vary, with some sources defining (W) as work done by the system, leading to the equation (ΔU=Q-W).
- Work (w): In an expansion or compression process,
- Heat (q): Energy transfer due to temperature difference.
- Internal Energy (U): Total energy stored within the system (includes kinetic and potential energy of particles).
Enthalpy (H)
Enthalpy (H) is a thermodynamic property that represents the total heat content of a system. It is defined as the sum of the system’s internal energy (U) plus the product of its pressure (P) and volume (V), expressed by the equation (H=U+PV). The change in enthalpy (ΔH) is particularly useful because it equals the heat absorbed or released in a process occurring at constant pressure, which is common for many chemical reactions and physical changes.
Types of Enthalpy Changes:
- ΔHₑₓₒ: Exothermic (heat released, negative ΔH)
- ΔHₑₙdₒ: Endothermic (heat absorbed, positive ΔH)
Hess’s Law of Constant Heat Summation
Hess’s Law of Constant Heat Summation states that the total enthalpy change for a chemical reaction is the same, regardless of the number of steps or the pathway taken, and depends only on the initial and final states of the reaction. This means the sum of the enthalpy changes of a series of reactions equals the overall enthalpy change of a single reaction. The law is a direct consequence of the principle of conservation of energy and the fact that enthalpy is a state function.
Applications:
- Calculating enthalpy of formation, combustion, or reaction.
- Determining lattice energy or bond enthalpy indirectly.
Enthalpy Changes in Chemical Reactions
- Heat of Formation (ΔHf): Enthalpy change when one mole of a compound is formed from its elements.
- Heat of Combustion (ΔHc): Enthalpy change when one mole of substance is completely burnt in oxygen.
- Heat of Neutralization: Enthalpy change when an acid reacts with a base to form water.
- Heat of Solution: Enthalpy change when one mole of solute dissolves in solvent.
- Heat of Atomization: Energy required to form atoms from a molecule.
Second Law of Thermodynamics
The Second Law of Thermodynamics states that the total entropy (disorder) of an isolated system can only increase over time, meaning that spontaneous processes always move from order to disorder. It can be expressed in several ways, such as that heat naturally flows from hotter to colder objects, and that not all heat can be converted into work in a cyclical process. This law explains why certain processes are irreversible and provides a limit to energy efficiency, impacting the design of engines and refrigerators.
- Irreversibility: Many natural processes are irreversible. For example, a broken cup will not spontaneously reassemble itself; the disorder (entropy) increases when the cup breaks, but it won’t spontaneously reverse to a state of lower entropy.
- Energy quality: The law implies that energy has “quality” as well as “quantity”. While the total amount of energy is conserved (first law), its quality (ability to do useful work) can decrease as it is converted into less useful forms like low-grade heat.
Gibbs Free Energy (G)
Gibbs free energy (G) is a thermodynamic potential that measures the maximum reversible work a system can perform at constant temperature and pressure. It is used to predict whether a process is spontaneous, based on the change in Gibbs free energy (ΔG). The formula for this change is (ΔG= ΔH – TΔS), where (ΔH) is enthalpy change, (T) is absolute temperature, and (ΔS) is entropy change.
Conditions for Spontaneity:
-
If ΔG < 0 → spontaneous process
-
If ΔG > 0 → non-spontaneous process
-
If ΔG = 0 → equilibrium
Relationship Between ΔG and Equilibrium Constant
The standard free energy change (ΔG°) is directly related to the equilibrium constant (K) by the equation ΔG°=-RTln K. This equation shows that a negative (ΔG°) corresponds to a large K (favoring products), a positive (ΔG°) corresponds to a small (K) (favoring reactants), and a (ΔG°) of zero corresponds to K=1.
Where:
- ΔG° = Standard Gibbs free energy change
- R = Gas constant
- T = Temperature (K)
- K = Equilibrium constant








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









