The National Eligibility cum Entrance Test is a single window exam conducted every year by the National Testing Agency at various exam centers across the nation to select students eligible for admission into various medical courses like MBBS and BDS. The exam, test student’s knowledge and understanding of important topics from Physics, Biology and Chemistry subjects. Chemical Kinetics is one such chapter from the NEET Chemistry Syllabus 2026 that all the aspirants need to study in process to complete their preparations for the NEET 2026 exam. Therefore, in this article, we have shared the NEET Notes for the Chemical Kinetics Chapter, scroll down in the article and access the notes.
Chemical Kinetics
Chemical kinetics is the study of chemical reaction rates, the factors that affect them (like temperature and concentration), and the reaction mechanisms by which they occur. It classifies reactions as fast, slow, or moderate and uses concepts like rate laws, reaction order, and collision theory to describe how reactions happen. Ultimately, it helps us understand and control how quickly a chemical change takes place.
To assist students with their exam preparation, we have shared the overview of all the important topics to be studied in this chapter. Scroll down in the article and start preparing:
1. Rate of Reaction
The rate of a reaction refers to the change in concentration of reactants or products per unit time.
Where,
- [R] = concentration of reactant
- [P] = concentration of product
= time
Unit: mol L⁻¹ s⁻¹
2. Average and Instantaneous Rate
-
Average Rate:
It gives the rate over a finite time interval.
-
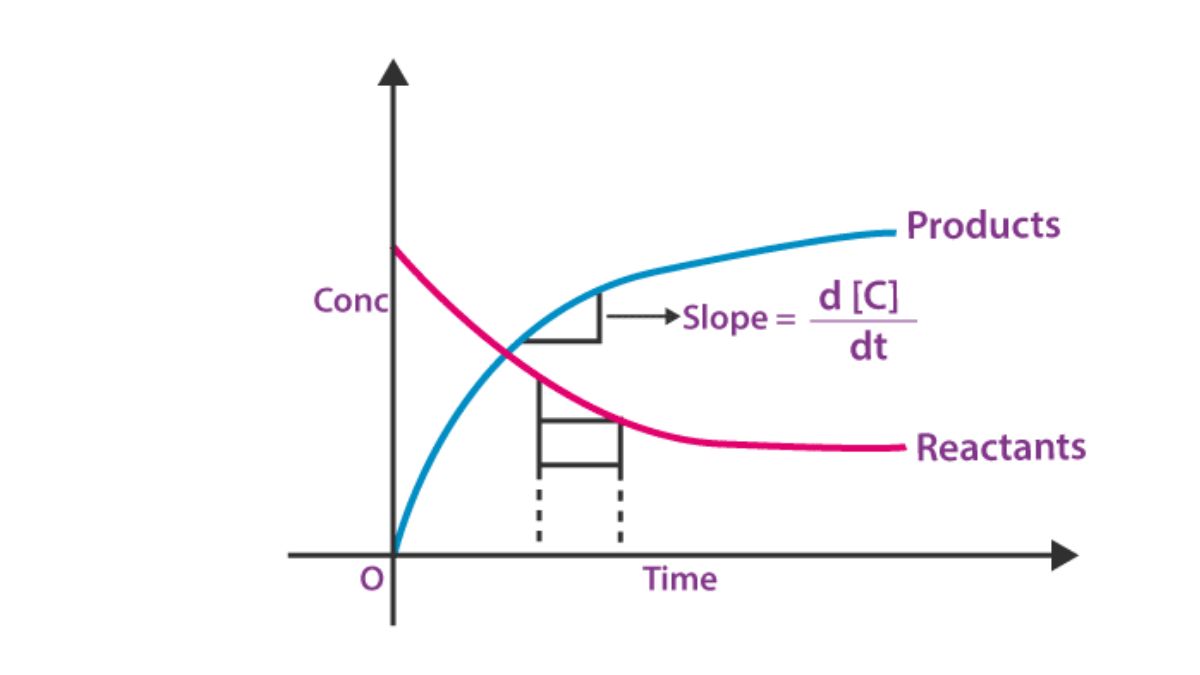
Instantaneous Rate:
It is the rate at a particular instant of time and is obtained from the slope of the concentration-time graph.
3. Factors Affecting the Rate of Reaction
- Concentration of Reactants – Higher concentration increases the rate due to more frequent collisions.
- Temperature – Increase in temperature increases kinetic energy, hence the reaction rate.
- Nature of Reactants – Ionic reactions occur faster than covalent ones.
- Presence of Catalyst – Catalyst lowers activation energy, increasing the rate.
- Surface Area – Greater surface area of reactants increases reaction rate.
- Pressure – In gaseous reactions, higher pressure increases reaction rate.
4. Rate Law and Rate Constant
The rate law expresses the relation between reaction rate and concentration of reactants.
For a reaction:
The rate law is:
Where,
-
= Rate constant
-
= Order of reaction with respect to A and B respectively
5. Order of a Reaction
The order of reaction is the sum of powers of concentration terms in the rate law:
Characteristics:
- Can be 0, 1, 2, fractional, or even negative.
- Determined experimentally.
- It is not related to stoichiometric coefficients.
6. Molecularity of a Reaction
The molecularity of a reaction is the number of reactant molecules that collide simultaneously to form products.
Examples:
- Unimolecular reaction: Decomposition of N₂O₅
- Bimolecular reaction: 2HI → H₂ + I₂
Note:
- Molecularity is always a whole number.
- It is a theoretical concept, unlike order which is experimental.
7. Integrated Rate Equations
(a) Zero Order Reaction
-
Half-life:
(b) First Order Reaction
-
Half-life:
-
Independent of initial concentration.
(c) Second Order Reaction
-
Half-life:
8. Arrhenius Equation
This equation shows how rate constant (k) varies with temperature.
Where,
-
= Frequency factor
-
= Activation energy
-
= Gas constant
-
= Temperature (K)
Logarithmic form:
Graph of
vs
gives a straight line with slope
.
9. Collision Theory
According to this theory:
- Reactants must collide for a reaction to occur.
- Collisions must have sufficient energy (≥ Eₐ) and proper orientation.
- Only effective collisions result in product formation.
10. Catalyst and Activation Energy
A catalyst speeds up a chemical reaction by providing an alternative pathway with a lower activation energy, which is the minimum energy needed for the reaction to occur. Activation energy is the energy barrier that reactant molecules must overcome to form products. Without a catalyst, more energy is required, and the reaction is slower; with a catalyst, this energy barrier is reduced, allowing more molecules to react and increasing the reaction rate.
Importance of Chemical Kinetics NEET Notes
Preparing for the Chemical Kinetics Chapter? Use the latest and updated NEET Notes shared by the expert faculty members at Adda247. Below, we have cited some of the pointers explainting the importance of Chemical Kinetics NEET Notes:
- Strengthens Conceptual Understanding: Chemical Kinetics helps students understand how and why reactions occur at specific rates, building a strong foundation for problem-solving in Physical Chemistry.
- Essential for Numerical Problem Solving: The topic involves key formulas such as rate laws, half-life, and Arrhenius equation, which are commonly used in NEET numerical questions.
- Helps Predict Reaction Behavior: Understanding reaction rates enables students to predict how changes in concentration, temperature, or catalysts affect reaction speed.
- Bridges Thermodynamics and Reaction Mechanisms: Kinetics complements thermodynamics by explaining the actual pathway and speed of chemical change, offering a complete picture of reactions.
- Improves Analytical and Graphical Skills: Students learn to interpret and analyze rate vs. concentration and ln k vs. 1/T graphs, which are frequently tested in NEET exams.
- Reinforces Real-life Applications: Concepts like catalysis, enzyme kinetics, and industrial reaction rates help students relate chemistry principles to everyday and biological processes.
- High-weightage Topic in NEET Chemistry: Every year, 2–3 direct questions appear from this topic, making it one of the most scoring and conceptual chapters in NEET Physical Chemistry.
- Foundation for Advanced Studies: Mastery of Chemical Kinetics is crucial for future topics in medicine, biochemistry, and chemical engineering, where reaction rate analysis is vital.
NEET Important Question – Chemical Kinetics
Question 1. A reaction between two gases is bimolecular. If pressure is doubled, what happens to the reaction rate?
Question 2. The decomposition of N₂O₅ follows first-order kinetics. If the half-life period is 100 s, calculate the rate constant.
Question 3. Explain the significance of the rate constant (k) in a chemical reaction.
Question 4. Explain why reactions with lower activation energy occur faster at a given temperature.
Question 5. State the difference between order of reaction and molecularity of reaction with suitable examples.
Question 6. The half-life of a zero-order reaction is 20 minutes when the initial concentration is 0.1 M. What will be the half-life if the initial concentration is 0.05 M?
Question 7. Why is the half-life of a first-order reaction independent of the initial concentration?
Question 8. Derive an expression for the rate constant of a second-order reaction in terms of concentration and time.
Question 9. Plot a graph between ln [A] vs time for a first-order reaction and explain its slope and intercept.
Question 10. Calculate the activation energy if the rate constant doubles when the temperature increases from 300 K to 310 K.
Question 11. Explain the Arrhenius equation and derive its logarithmic form.
Question 12. For a reaction 2A + B → Products, the rate = k[A]²[B]. What is the overall order of the reaction?
Question 13. Define the rate of reaction and explain the difference between average rate and instantaneous rate.
Question 14. A reaction has an activation energy of 50 kJ mol⁻¹. What happens to the rate of reaction if the temperature is increased by 10°C?
Question 15. Write the rate law for a general reaction and define its terms.
Question 16. What is activation energy? How does it affect the rate of reaction?
Question 17. Why is molecularity always a whole number but order can be fractional or zero?
Question 18. How does the collision theory explain the effect of temperature on reaction rate?
Question 19. What is the difference between zero order and first order reactions?
Question 20. For a reaction A → B, the concentration of A decreases from 1.0 mol L⁻¹ to 0.25 mol L⁻¹ in 20 minutes. Show that it follows first-order kinetics.
Question 21. What factors influence the rate of a chemical reaction? Explain with examples.
Question 22. The rate constant for a first-order reaction is 5 × 10⁻³ s⁻¹. Calculate the time required for 75% of the reaction to complete.
Question 23. How does a catalyst alter the rate of a reaction without being consumed?








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









