The National Eligibility cum Entrance Test for admission into medical courses like MBBS and BDS is expected to be conducted by the National Testing Agency at various exam centers across the nation in May 2026. All the aspirants who are to appear for the admission must start preparing for the exam by reading all the topics mentioned in the NEET Syllabus 2026.
The NEET Chemistry Syllabus 2026 mentions Chemical Equilibrium as one of the important chapters that need to be studied by the aspirants in process to complete their exam preparations. In the article below, aspirants can find out the NEET Notes for Chemical Equilibrium along with the important questions to complete exam preparations.
Chemical Equilibrium
Chemical equilibrium is the state in a reversible reaction where the forward and reverse reactions occur at the same rate, resulting in constant concentrations of reactants and products. This is a dynamic state, meaning reactions are still happening at the molecular level, but there is no net change observed in the system’s properties like concentration, pressure, or color. The equilibrium is defined by the equilibrium constant (
), which shows the ratio of product to reactant concentrations at a specific temperature.
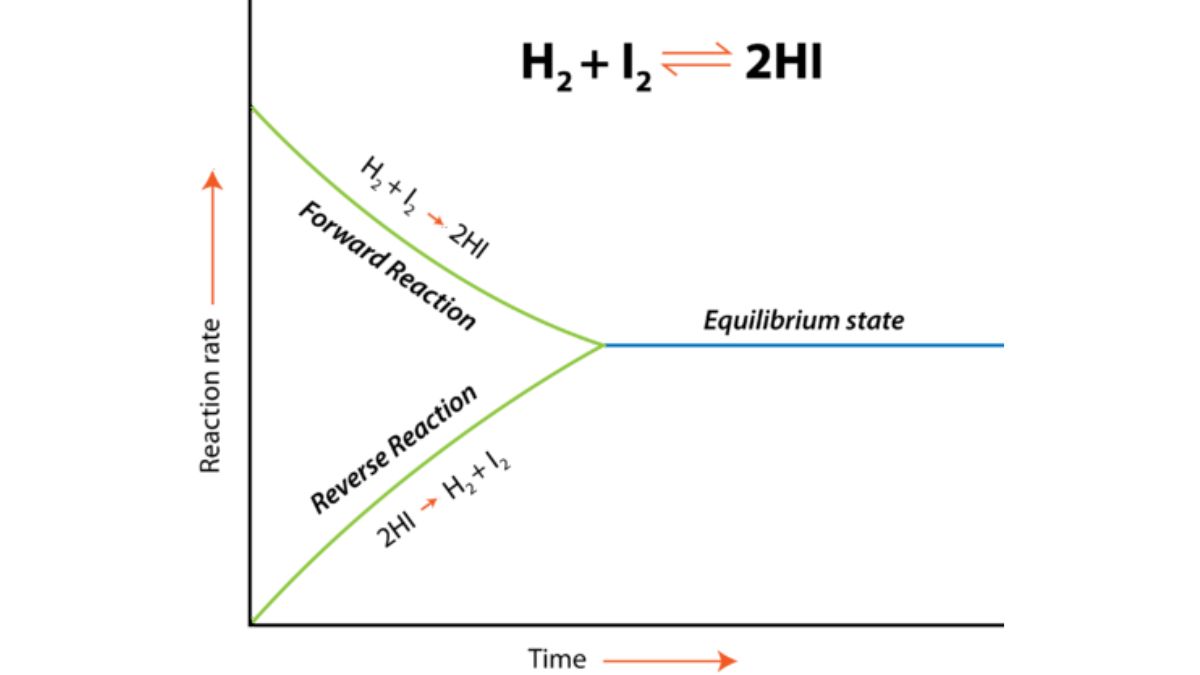
Reversible and Irreversible Reactions
- A reversible reaction is a chemical process that proceeds in both forward and backward directions simultaneously, meaning reactants form products, and products can reform the original reactants. When the rates of these two opposing reactions become equal, the system reaches a state of dynamic equilibrium, where the concentrations of reactants and products remain constant, even though both reactions continue to occur.
Example:
-
Irreversible reactions are one-way chemical processes where reactants are completely converted into products, and the products cannot revert back to the original reactants under the same conditions. These reactions are represented by a single arrow (→) and go to completion, meaning all reactants are consumed. Examples include cooking an egg, burning paper, and combustion.
Dynamic Nature of Equilibrium
The dynamic nature of equilibrium describes a state in a reversible reaction where the rate of the forward reaction is equal to the rate of the reverse reaction, resulting in no observable change in the concentrations of reactants and products. Despite the constant concentrations, the reaction is not static; individual molecules continue to react in both directions simultaneously, which is why it is called “dynamic.”
Law of Mass Action
The law of mass action states that the rate of a chemical reaction is proportional to the product of the active masses (concentrations) of the reactants, each raised to the power of its stoichiometric coefficient. Formulated by Guldberg and Waage, it also provides an expression for the equilibrium constant, which is the ratio of the concentrations of products to reactants at equilibrium. The law is based on the principle that reactions occur through molecular collisions, so more reactant molecules mean more collisions and a faster reaction rate.
For a general reaction:
The equilibrium constant is given by:
Equilibrium Constant (Kc and Kp)
-
Kc → Concentration-based constant (mol/L).
-
Kp → Pressure-based constant (used for gaseous systems).
Relation between the two:
where
Characteristics of Equilibrium Constant
The equilibrium constant (K) is a value that represents a reaction’s state at equilibrium and has key characteristics: it depends on temperature, is independent of initial concentrations, and indicates the direction of the reaction. Its value changes with temperature, but a catalyst does not affect it. For a given reaction, the constant has a specific value at a specific temperature and is the reciprocal of the constant for the reverse reaction.
- It is temperature-dependent.
- It does not depend on initial concentrations or pressure.
- A large
means the reaction is product-favored; a small
means reactant-favored.
Le Chatelier’s Principle
Le Chatelier’s Principle states that if a system at equilibrium is subjected to a change in concentration, temperature, or pressure, the system will shift in a way that counteracts the change to establish a new equilibrium. For example, increasing the concentration of a reactant will cause the equilibrium to shift toward the products, while increasing the pressure on a gas-phase reaction will favor the side with fewer moles of gas. A catalyst does not affect the position of the equilibrium, only the time it takes to reach it.
Applications:
- Increase in concentration of reactants: Equilibrium shifts toward products.
- Increase in pressure: Favors the side with fewer gas molecules.
- Increase in temperature: Favors the endothermic direction.
Factors Affecting Equilibrium
- Concentration – Changing reactant or product concentration disturbs equilibrium.
- Pressure – Affects gaseous reactions with different moles of reactants and products.
- Temperature – Alters
; equilibrium shifts to favor endothermic or exothermic side.
- Catalyst – Speeds up both forward and reverse reactions equally; does not affect equilibrium position.
Ionic Equilibrium and Solubility Product
Ionic equilibrium describes the dynamic balance between ions and undissolved molecules in a solution of a weak electrolyte or a sparingly soluble salt. The solubility product (
) is a specific type of equilibrium constant for a sparingly soluble ionic compound, representing the maximum product of the molar concentrations of its constituent ions in a saturated solution at a given temperature. Comparing the ionic product (IP), the product of ion concentrations in any solution, to the (
) helps predict whether a precipitate will form: if IP>
, precipitation occurs; if IP<
, more salt can dissolve; and if IP=
, the solution is saturated at equilibrium.
Relationship Between Reaction Quotient (Q) and K
The reaction quotient (Q) and the equilibrium constant (K) are used to determine the direction of a reversible chemical reaction. Comparing their values indicates the system’s state: if Q=K, the system is at equilibrium; if Q<K, the reaction will proceed forward to favor products; and if Q>K, the reaction will proceed in reverse to favor reactants.
- Q < K → Reaction moves forward.
- Q > K → Reaction moves backward.
- Q = K → System is at equilibrium.
NEET Important Questions – Chemical Equilibrium
Question 1. What is meant by chemical equilibrium? Why is it called a dynamic equilibrium?
Question 2. State the Law of Mass Action and explain its significance in chemical equilibrium.
Question 3. What conditions are necessary for a chemical reaction to attain equilibrium?
Question 4. Differentiate between homogeneous and heterogeneous equilibrium with suitable examples.
Question 5. Why does the equilibrium constant
change with temperature but not with concentration or pressure?
Question 6. Explain how Le Chatelier’s Principle predicts the effect of pressure on the synthesis of ammonia in the Haber process.
Question 7. How does the addition of an inert gas at constant pressure affect the position of equilibrium?
Question 8. What happens to the equilibrium position when a catalyst is added to a reversible reaction?
Question 9. Define the reaction quotient (Q) and explain its relationship with the equilibrium constant (K).
Question 10. What is the significance of the magnitude of
for a chemical reaction?
Question 11. Explain the role of temperature and pressure in maximizing the yield of ammonia during the Haber process.
Question 12. How does Le Chatelier’s Principle apply to the dissociation of CaCO₃ in a closed system?
Question 13. Why does heating an equilibrium mixture of
and
favor the formation of more
?
Question 14. Discuss how Gibbs free energy (
) is related to the equilibrium constant
.
Question 15. What is the difference between the solubility product (Ksp) and the ionic product (Qsp)? How are they used to predict precipitation?








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









