The preparations for the NEET Exam require aspirants to start learning for all the important topics mentioned in the NEET Syllabus 2026. The syllabus has been divided into three major subjects, i.e., Biology, Chemistry, and Physics. Each subject comprises of numerous chapters that need to be studied by all the aspirants.
One of the key chapters that all the aspirants need to study from the NEET Biology Syllabus 2026 is Biomolecules. Therefore, in this article, we have shared the NEET Notes for the Biomolecules Chapter. Scroll down in the article below and access the PDF.
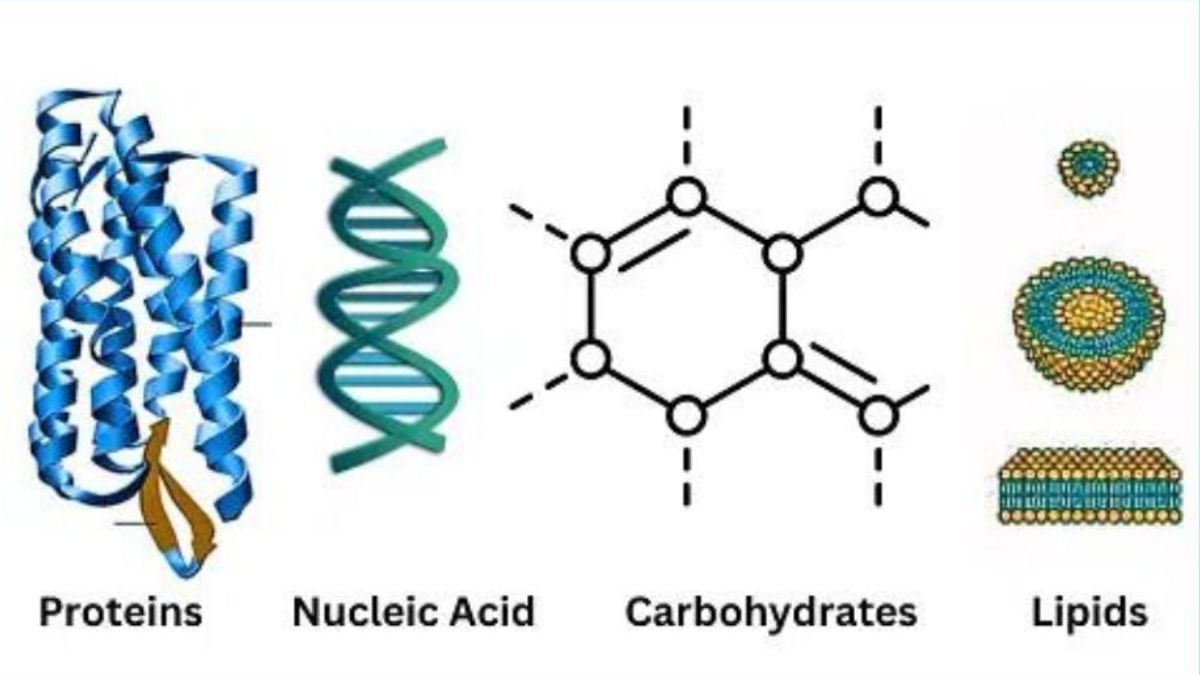
Biomolecules
Biomolecules are organic molecules produced by living organisms that are essential for life’s functions and processes. Biomolecules focuses on the structure, function, and examples of the major organic molecules in living organisms, namely carbohydrates, proteins, lipids, and nucleic acids. Key concepts covered in this chapter are micromolecules and macromolecules, understanding the chemical properties and structures of each type (e.g., monosaccharides, amino acids, polypeptide chains), and learning about their roles in biological processes.
Biomolecules NEET Notes Class 11
Biomolecules are present in all living organisms and are responsible for the structure, function, and regulation of cells, tissues, and organs. For NEET aspirants, understanding biomolecules is essential as it forms the foundation of molecular biology and biochemistry. Below, we have listed an overview of all the important topics covered in the Biomolecules Chapter of NEET Biology:
1. Introduction to Biomolecules
Biomolecules are broadly classified into organic and inorganic biomolecules.
- Organic Biomolecules: Contain carbon as the primary element along with hydrogen, oxygen, nitrogen, and sometimes sulfur and phosphorus. Examples: Carbohydrates, Proteins, Lipids, Nucleic Acids, Vitamins.
- Inorganic Biomolecules: Do not primarily contain carbon. Examples: Water, Minerals, Electrolytes.
Water constitutes about 70–80% of the living cell and is crucial for all biochemical reactions.
2. Classification of Biomolecules
A. Carbohydrates
Definition: Organic compounds composed of carbon, hydrogen, and oxygen, generally with the empirical formula (CH₂O)n.
Functions:
- Provide energy (glucose → ATP)
- Serve as structural components (cellulose in plants, chitin in arthropods)
- Act as signaling molecules (glycoproteins)
Types of Carbohydrates:
- Monosaccharides: Simple sugars (glucose, fructose, galactose)
- Disaccharides: Two monosaccharides (sucrose, lactose, maltose)
- Oligosaccharides: 3–10 monosaccharides
- Polysaccharides: Many monosaccharides (starch, glycogen, cellulose, chitin)
B. Proteins
Definition: Polymers of amino acids linked by peptide bonds. Proteins are vital for the structure and functioning of cells.
Functions:
- Structural: Collagen, Keratin
- Enzymatic: Amylase, Lipase
- Hormonal: Insulin, Glucagon
- Transport: Hemoglobin, Myoglobin
- Defense: Antibodies (Immunoglobulins)
- Storage: Ferritin, Casein
Levels of Protein Structure:
- Primary – Amino acid sequence
- Secondary – α-helix, β-sheet (hydrogen bonding)
- Tertiary – 3D folding
- Quaternary – Multiple polypeptide chains
C. Lipids
Definition: Hydrophobic molecules, primarily composed of carbon, hydrogen, and a small amount of oxygen.
Functions:
- Energy storage (triglycerides)
- Cell membrane structure (phospholipids)
- Insulation and protection
- Hormone precursors (steroids)
Types of Lipids:
- Fats and Oils (Triglycerides)
- Phospholipids (Cell membrane components)
- Steroids (Cholesterol, Hormones)
- Waxes (Protective coating)
D. Nucleic Acids
Definition: Biomolecules responsible for storing and transmitting genetic information. Composed of nucleotides (Nitrogenous base + Sugar + Phosphate group).
Types:
- DNA (Deoxyribonucleic Acid) – Genetic material
- RNA (Ribonucleic Acid) – Protein synthesis
Functions:
- Storage of genetic information
- Transmission of hereditary traits
- Regulation of cell activities
- Synthesis of proteins
E. Vitamins
Definition: Organic compounds required in small quantities for proper metabolic function.
Classification:
- Water-soluble: Vitamin B complex, Vitamin C
- Fat-soluble: Vitamins A, D, E, K
Functions: Act as coenzymes and antioxidants, support growth, vision, blood clotting, and bone health.
F. Minerals
Definition: Inorganic elements required in small quantities for physiological functions.
Major Minerals: Calcium, Phosphorus, Magnesium, Sodium, Potassium
Trace Elements: Iron, Iodine, Zinc, Copper
Functions:
- Structural roles (bones, teeth)
- Cofactors for enzymes
- Electrolyte balance
- Oxygen transport (Iron in hemoglobin)
G. Water
Importance of Water:
- Medium for biochemical reactions
- Solvent for nutrients
- Temperature regulation
- Transport of substances
3. Tests for Biomolecules
| Tests for Biomolecules | ||
| Biomolecule | Test | Positive Result |
| Carbohydrates | Benedict’s test |
Red/Orange (Reducing sugar)
|
| Starch | Iodine test | Blue-Black |
| Proteins | Biuret test | Violet |
| Lipids | Sudan III |
Red-stained oil layer
|
| Nucleic acids | Dische Diphenylamine | Blue (DNA) |
Importance of Biomolecules NEET Notes Class 11
The Biomolecules NEET Notes Class 11 are a very crucial factor for preparation; therefore, we have shared a list of reasons dictating the importance of Biomolecules NEET Notes Class 11:
- Biomolecules are the chemical basis of life, and understanding them helps students connect physiology, genetics, and cell biology.
- Many direct and application-based questions are asked from carbohydrates, proteins, lipids, nucleic acids, and enzymes, making it a scoring topic.
- Enzymes, their properties, and factors affecting them are frequently tested in NEET, so clear notes help in quick revisions.
- Notes simplify the learning of important structures (DNA, RNA, peptide bonds, polysaccharides) with relevant examples.
- MCQs based on biochemical pathways, structural differences, and functions can be easily solved if the notes are well-prepared.
- Biomolecules link with physiology, genetics, and metabolism, making it essential for a better understanding of advanced biology.
- Well-structured NEET notes allow students to revise important formulas, diagrams, and key differences in less time before the exam.
NEET Important Question – Biomolecules
Question 1. Which of the following is a disaccharide
a) Glucose
b) Fructose
c) Maltose
d) Galactose
Question 2. The bond present between two monosaccharides in a disaccharide is:
a) Hydrogen bond
b) Glycosidic bond
c) Peptide bond
d) Ester bond
Question 3. The storage form of carbohydrate in plants is:
a) Glycogen
b) Cellulose
c) Starch
d) Maltose
Question 4. Which vitamin is essential for blood clotting?
a) Vitamin A
b) Vitamin C
c) Vitamin D
d) Vitamin K
Question 5. In a nucleotide, the nitrogenous base is linked to sugar by:
a) Glycosidic bond
b) Phosphodiester bond
c) Peptide bond
d) Hydrogen bond
Question 6. The secondary structure of proteins is maintained by:
a) Hydrogen bonds
b) Peptide bonds
c) Disulfide bonds
d) Ionic bonds
Question 7. Which lipid forms the structural component of cell membrane?
a) Steroids
b) Triglycerides
c) Phospholipids
d) Glycolipids
Question 8. Enzyme that hydrolyzes starch into maltose is:
a) Amylase
b) Lipase
c) Maltase
d) Sucrase
Question 9. Differentiate between DNA and RNA in terms of sugar and nitrogenous bases.
Question 10. Why are lipids considered hydrophobic in nature?
Question 11. Name two examples of essential amino acids.
Question 12. State the role of hemoglobin as a protein.
Question 13. What type of bond joins two amino acids?
Question 14. Which polysaccharide is the major component of plant cell walls?
Question 15. Name the vitamin whose deficiency leads to night blindness.
Question 16. A person suffers from delayed blood clotting. Which vitamin deficiency might be the cause?
Question 17. If a DNA molecule has 20% adenine, calculate the percentage of guanine.
Question 18. Why is cellulose not digested by humans, though it is a polysaccharide?
Question 19. What structural difference makes glycogen more suitable for energy storage than starch?
Question 20. Why are enzymes called “biological catalysts”? Give two examples.










 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...








