The NEET 2026 exam is to be conducted by the National Testing Agency at various exam centers across the nation in May 2026. All the aspirants of this exam who are currently studying in class 12th or have taken a year off for exam preparations must read and learn all the important topics and chapters mentioned in the NEET Syllabus 2026. The Amines chapter is a crucial part of the NEET Chemistry Syllabus 2026; therefore, it is crucial that all the aspirants must complete their preparations for this chapter by scrolling down in the article and using the best in class NEET Notes for the Amines Chapter.
What Are Amines?
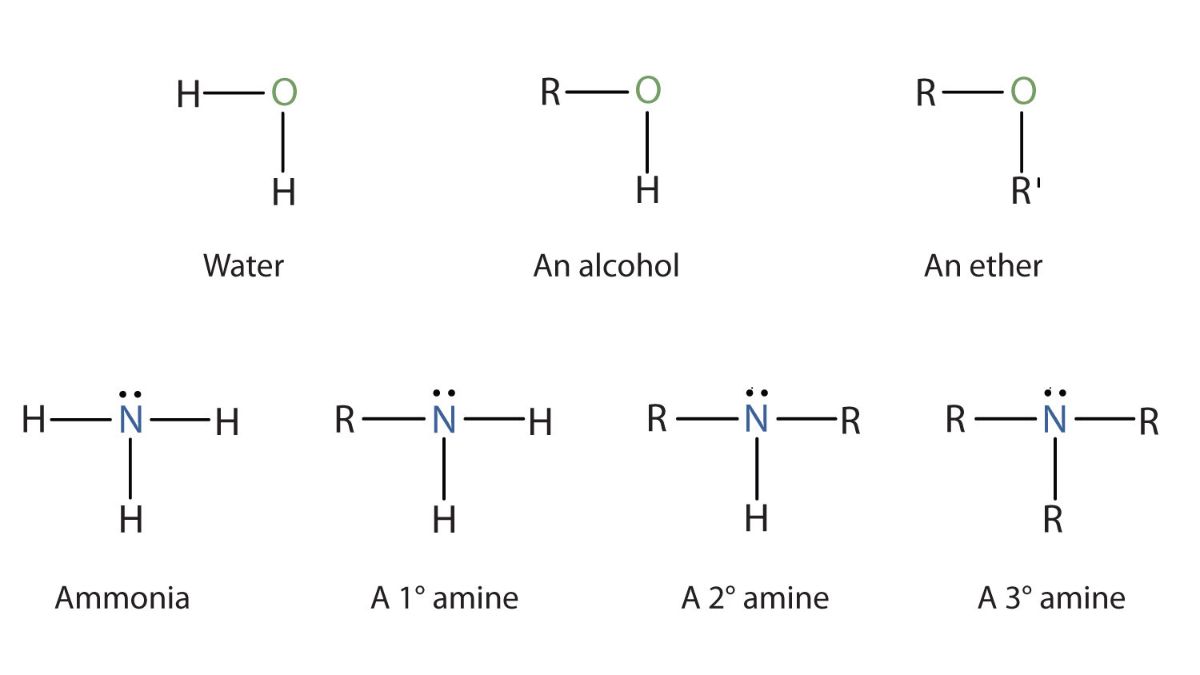
Amines are organic compounds derived from ammonia (\(NH_{3}\)) where one or more hydrogen atoms are replaced by alkyl or aryl groups. They are classified as primary, secondary, and tertiary based on the number of hydrogen atoms replaced. Amines are basic, have a lone pair of electrons on the nitrogen atom, and are vital components of natural substances like proteins, amino acids, and vitamins.
Amines are organic compounds that contain nitrogen atoms bonded to alkyl or aryl groups. They can be considered as derivatives of ammonia, where one or more hydrogen atoms are replaced by alkyl or aryl groups.
Below, we have shared an overview of all the important topics covered in the Amines Chapter of NEET Chemistry Syllabus 2026. Scroll down and start preparing:
General Formula:
- For aliphatic amines: CₙH₂ₙ₊₃N
- For aromatic amines: Derived from aromatic hydrocarbons such as benzene.
Classification of Amines
Amines are classified based on the number of hydrogen atoms replaced by alkyl or aryl groups:
- Primary amine (1°): One hydrogen atom of NH₃ is replaced.
Example: CH₃NH₂ (Methylamine) - Secondary amine (2°): Two hydrogen atoms of NH₃ are replaced.
Example: (CH₃)₂NH (Dimethylamine) - Tertiary amine (3°): All three hydrogen atoms of NH₃ are replaced.
Example: (CH₃)₃N (Trimethylamine)
Nomenclature of Amines
Common System
-
The alkyl or aryl group is named first, followed by the word “amine.”
Example: CH₃NH₂ → Methylamine
IUPAC System
- The longest carbon chain attached to nitrogen is taken as the parent chain.
- The suffix “-amine” is added to the parent chain name.
Example: CH₃CH₂NH₂ → Ethanamine
For secondary and tertiary amines, substituents on nitrogen are indicated by the prefix ‘N-’.
Example: N-Methylmethanamine
Structure of Amines
- The nitrogen atom in amines is sp³ hybridized.
- The geometry around nitrogen is trigonal pyramidal.
- Due to the presence of a lone pair, amines can form hydrogen bonds.
Preparation of Amines
-
Reduction of Nitro Compounds:
R–NO₂ → R–NH₂
(Using Sn/HCl or catalytic hydrogenation) -
Ammonolysis of Alkyl Halides:
R–X + NH₃ → R–NH₂ + HX -
Reduction of Nitriles:
R–C≡N + 2H₂ → R–CH₂NH₂ -
Reduction of Amides:
R–CONH₂ → R–CH₂NH₂ (Using LiAlH₄) -
Gabriel Phthalimide Synthesis:
Produces pure primary amines.
Phthalimide → Alkyl Phthalimide → Alkylamine -
Hofmann Bromamide Degradation:
Converts amides to primary amines with one carbon less.
R–CONH₂ + Br₂ + KOH → R–NH₂ + CO₂
Physical Properties of Amines
- Lower aliphatic amines are gases with fishy odour.
- Higher amines are liquids or solids.
- Amines can form hydrogen bonds — hence, primary and secondary amines are soluble in water.
- Boiling point order: Primary > Secondary > Tertiary
(Due to hydrogen bonding)
Chemical Properties of Amines
-
Basic Character:
-
Amines act as bases due to the lone pair of electrons on nitrogen.
-
Basicity order:
Aliphatic amines > Ammonia > Aromatic amines
-
-
Acylation:
-
Reaction with acid chlorides or anhydrides forms amides.
Example: RNH₂ + CH₃COCl → RNHCOCH₃
-
-
Alkylation:
-
Amines react with alkyl halides to form higher amines.
-
-
Carbylamine Reaction (for primary amines):
RNH₂ + CHCl₃ + KOH → R–NC + 3KCl + 3H₂O
(Foul-smelling isocyanides are formed) -
Diazotization (for aromatic primary amines):
ArNH₂ + NaNO₂ + HCl → ArN₂⁺Cl⁻ + 2H₂O
(Forms diazonium salt) -
Hinsberg Test:
-
Used to distinguish between primary, secondary, and tertiary amines.
-
Aromatic Amines (Aniline and Derivatives)
Aniline (C₆H₅NH₂) is the simplest aromatic amine.
It is obtained by the reduction of nitrobenzene.
Reactions of Aniline:
- Acylation: Forms acetanilide.
- Bromination: Forms 2,4,6-tribromoaniline.
- Diazotization: Forms benzenediazonium chloride.
Uses of Amines
- Used in the synthesis of dyes, drugs, and polymers.
- Important in manufacturing explosives (TNT).
- Act as intermediates in the production of pesticides and pharmaceuticals.
NEET Important Questions – Amines
Question 1. How can primary, secondary, and tertiary amines be distinguished experimentally?
Question 2. How can diazonium salts be used to prepare azo dyes?
Question 3. Write the reaction for diazotization of aniline.
Question 4. Give the structure of product obtained from methylamine and acetyl chloride.
Question 5. Write the steps to synthesize methylamine from methanol.
Question 6. Explain the Hinsberg test and its significance.
Question 7. Write the chemical equation for the preparation of aniline using Gabriel synthesis.
Question 8. Why is tertiary amine less basic in water than secondary amine?
Question 9. Explain why aniline does not undergo Friedel–Crafts reaction easily.
Question 10. Convert acetonitrile to ethylamine.
Question 11. Explain the basic nature of amines using the Lewis concept.
Question 12. Differentiate between aliphatic and aromatic amines.
Question 13. Convert benzene to benzylamine.
Question 14. How can methylamine be prepared from acetamide?
Question 15. Convert nitrobenzene to aniline in two steps.
Question 16. Explain Gabriel Phthalimide synthesis.
Question 17. What happens when aniline reacts with bromine water?
Question 18. What are amines? Give their classification with examples.
Question 19. How will you convert nitroethane to ethylamine?
Question 20. Compare the boiling points of ethylamine and triethylamine.
Question 21. How can you prepare aniline from benzamide?
Question 22. What is the product when aniline is treated with acetic anhydride?
Question 23. Convert ethylamine to diethylamine.
Question 24. How will you prepare propylamine from propanol?
Question 25. What is the hybridization and geometry around nitrogen in amines?
Question 26. Which type of amine gives positive Carbylamine test?
Question 27. Why do amines have higher boiling points than alkanes of similar molecular mass?
Question 28. Write the mechanism of Hofmann Bromamide degradation.
Question 29. Why is aniline less basic than methylamine?
Question 30. Write the reaction for reduction of nitriles to amines.
Question 31. Write the chemical equation for the reduction of nitrobenzene to aniline.
Question 32. What happens when benzene diazonium chloride is treated with phenol?
Question 33. Name the reaction used to convert primary amides into primary amines.








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









