While preparing for the NEET 2026 exam, all the aspirants need to read and learn crucial topic covered in the NEET Syllabus 2026. All the students who are studying in class 12th or have took year off for exam preparation must now start preparing for the exam by reading all the important chapters mentioned in the exam syllabus. To assist students with the exam preparation, we are sharing NEET Notes for all the important chapters, therefore, in this article, we have shared the NEET Notes for the Alcohol, Ether and Phenol Chapter. Scroll down in the article and get the perfect notes.
Alcohol Ether and Phenol
Alcohols, ethers, and phenols are all organic compounds containing an oxygen atom, but they differ in the groups attached to the oxygen. Alcohols have a hydroxyl (-OH) group bonded to a saturated carbon atom (R-OH). Phenols have the -OH group directly bonded to an aromatic carbon ring (Ar-OH). Ethers have an oxygen atom linking two hydrocarbon groups, which can be alkyl or aryl (R-O-R’).
Below, we have shared an overvew of the Alcohol, Ether and Phenol Chapter so that all the aspirants can read and complete their preparations of the chapter using it.
1. Classification and Nomenclature
Alcohols
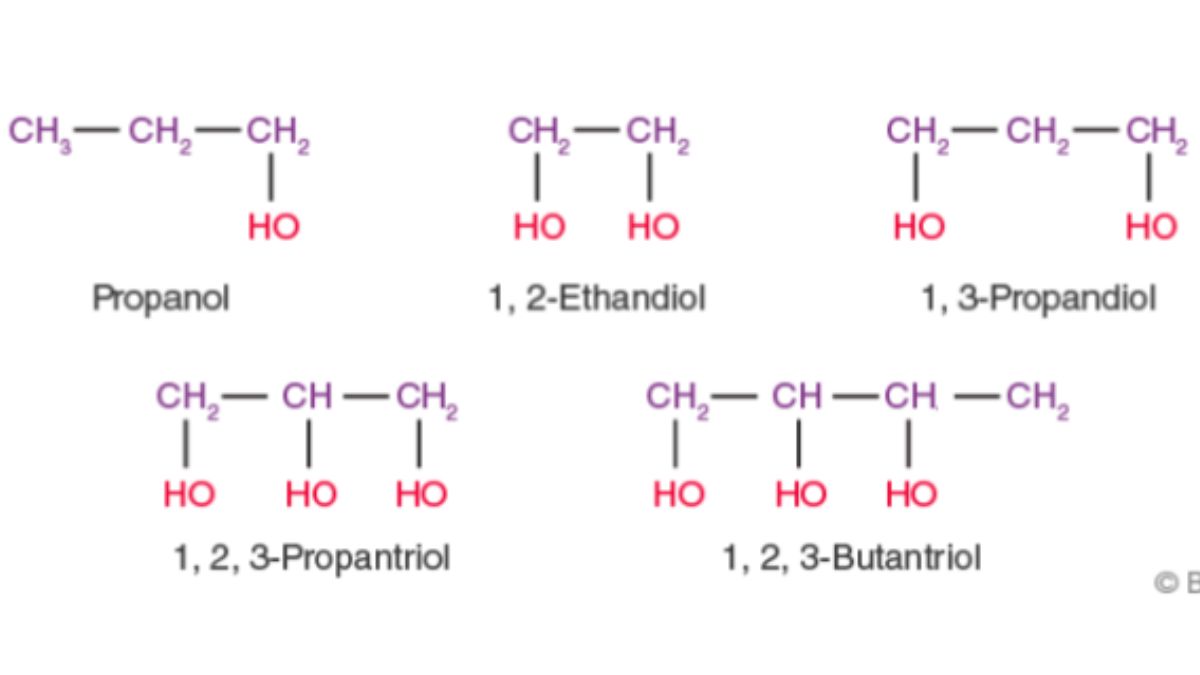
- General formula: CₙH₂ₙ₊₁OH
- Functional group: –OH (hydroxyl group)
- Classification:
- Primary (1°): –OH group attached to a carbon bonded to one alkyl group (e.g., ethanol).
- Secondary (2°): –OH group attached to a carbon bonded to two alkyl groups (e.g., isopropanol).
- Tertiary (3°): –OH group attached to a carbon bonded to three alkyl groups (e.g., tert-butanol).
Phenols
- General formula: Ar–OH
- Hydroxyl group directly attached to an aromatic ring.
- Example: Phenol (C₆H₅OH).
Ethers
- General formula: R–O–R′
- Functional group: –O– (alkoxy group)
- Symmetrical ethers (R = R′) and asymmetrical ethers (R ≠ R′).
- Example: Diethyl ether (CH₃CH₂–O–CH₂CH₃).
2. Preparation of Alcohols, Phenols, and Ethers
Preparation of Alcohols
- From Alkenes:
- Hydration: Ethene + H₂O → Ethanol (using acid catalyst).
- Hydroboration–oxidation: Anti-Markovnikov addition.
- From Carbonyl Compounds:
- Aldehydes + NaBH₄/LiAlH₄ → Primary alcohols.
- Ketones + NaBH₄/LiAlH₄ → Secondary alcohols.
- From Haloalkanes:
- R–X + KOH (aqueous) → R–OH
Preparation of Phenols
- From Chlorobenzene:
- C₆H₅Cl + NaOH (fused) → C₆H₅OH (Dow’s process).
- From Benzene Sulphonic Acid:
- C₆H₅SO₃H + NaOH → C₆H₅OH + Na₂SO₃
Preparation of Ethers
-
By Williamson Synthesis:
-
- R–ONa + R′–X → R–O–R′ + NaX
- Example: C₂H₅ONa + CH₃I → CH₃–O–C₂H₅
3. Physical Properties
| Physical Properties | |||
| Property | Alcohols | Phenols | Ethers |
| Hydrogen Bonding | Strong | Strong | Absent |
| Boiling Point | High | High | Low |
| Solubility in Water | High (low M.W.) | Moderate | Slight |
| Odour | Pleasant | Medicinal | Ether-like |
4. Chemical Properties
(a) Reactions of Alcohols
-
Oxidation:
-
Primary alcohol → Aldehyde → Acid
-
Secondary alcohol → Ketone
-
Tertiary alcohol → No oxidation
-
-
Dehydration:
-
Alcohols → Alkenes (using conc. H₂SO₄ and heat).
-
-
Reaction with Na:
-
2ROH + 2Na → 2RONa + H₂↑
-
-
Esterification:
-
ROH + R′COOH → R′COOR + H₂O
-
(b) Reactions of Phenols
-
Acidic Nature:
-
Phenol + Na → Sodium phenoxide + H₂↑
-
Phenol is more acidic than alcohol due to resonance stabilization of phenoxide ion.
-
-
Electrophilic Substitution:
-
Bromination: Phenol + Br₂ → 2,4,6-tribromophenol
-
Nitration: Phenol + HNO₃ → o-nitrophenol + p-nitrophenol
-
-
Kolbe’s Reaction:
-
Phenol + CO₂ + NaOH → Salicylic acid
-
-
Reimer–Tiemann Reaction:
-
Phenol + CHCl₃ + NaOH → Salicylaldehyde
-
(c) Reactions of Ethers
-
Cleavage by HI or HBr:
-
R–O–R′ + HX → R–X + R′–OH (or R′–X)
-
-
Autooxidation:
-
Ethers form peroxides on long exposure to air.
-
5. Distinguishing Tests
-
Lucas Test:
Differentiates between 1°, 2°, and 3° alcohols using conc. HCl + ZnCl₂.-
3° → Immediate turbidity
-
2° → Slow turbidity
-
1° → No turbidity at room temperature
-
-
Ferric Chloride Test:
Phenol gives violet coloration with neutral FeCl₃ solution.
6. Uses and Applications
- Alcohols: Used as fuel (ethanol), solvent, and antiseptic (isopropanol).
- Phenols: Used in antiseptics (Dettol), disinfectants, and synthesis of dyes.
- Ethers: Used as anesthetics (diethyl ether) and solvents.
NEET Important Questions – Alcohol, Ether, and Phenol
- Interconversion Reactions: Boosts problem-solving skills in organic chemistry.
- What are the products formed when phenol reacts with bromine water?
- Differentiate between alcohols, phenols, and ethers based on their structure.
- Why is phenol more acidic than ethanol?
- Frequent NEET Topic: 2–3 questions appear every year from this unit.
- Why do ethers have lower boiling points than alcohols?
- Concept Building: Simplifies complex organic reactions and mechanisms.
- What happens when ethanol reacts with concentrated sulfuric acid at 443 K?
- Mechanism Clarity: Helps in understanding oxidation, substitution, and elimination reactions.
- Exam Readiness: Short, formula-based revision for NEET preparation.
- Write a chemical equation for the preparation of diethyl ether using Williamson synthesis.
- Practical Understanding: Covers laboratory tests and real-life uses.
- Scoring Topic: Conceptual and numerical questions are generally direct.
- Explain the Kolbe’s reaction with an example.
- Foundation for Higher Topics: Basis for understanding carbonyl and carboxylic compounds.
- Write the mechanism of the Reimer–Tiemann reaction.
- Arrange the following in increasing order of acidity: water, ethanol, phenol.
- How does tertiary alcohol react with Lucas reagent?








 NTA NEET 2026 Notification OUT at neet.n...
NTA NEET 2026 Notification OUT at neet.n...
 NEET Preparation Strategy 2026: Detailed...
NEET Preparation Strategy 2026: Detailed...
 Free NEET Sample Papers 2026 PDF | Downl...
Free NEET Sample Papers 2026 PDF | Downl...









